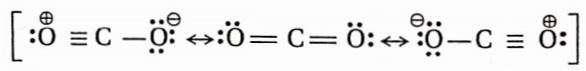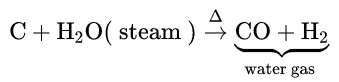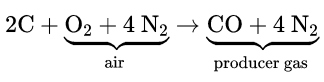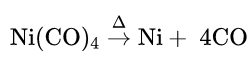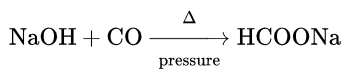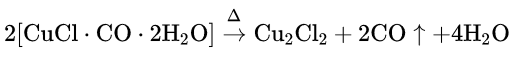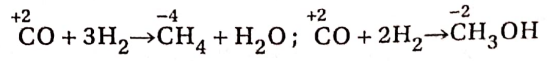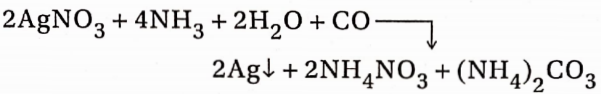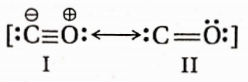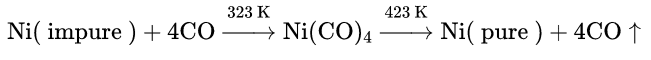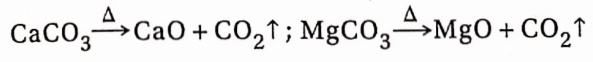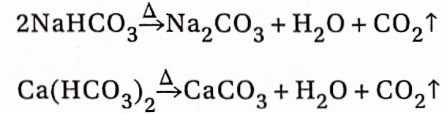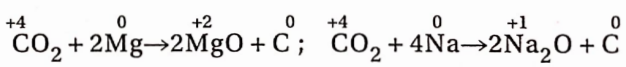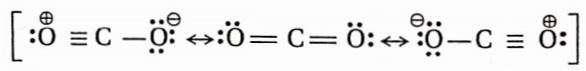Carbon forms three oxides,: e.g., carbon monoxide (CO), carbon dioxide (C02) and carbon suboxide (C3O2). Among these, the first two are important

1. Carbon Monoxide
Carbon Monoxide Laboratory preparation:
1. In the laboratory, carbon monoxide is prepared by dehydrating formic acid or oxalic acid after heating with concentrated sulphuric acid.

2. When potassium ferrocyanide is heated with excess of the cone, sulphuric acid, and pure carbon monoxide is obtained

CO cannot be dried by concentrated sulphuric acid:
Concentrated sulphuric acid is a strong oxidising agent. Thus, when CO (a reducing agent) is passed through concentrated H2SO4, it is oxidised by sulphuric acid to CO2
Read and Learn More CBSE Class 11 Chemistry Notes
Carbon Monoxide Other methods of preparation
1. From carbon:
When steam is passed over red hot coke, water gas or synthesis gas (CO + H2) is obtained. CO is separated from the mixture by liquefaction.
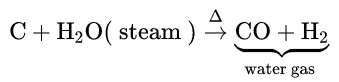
When air is passed over hot coke, producer gas (CO + N2) is formed. CO is separated by liquefaction
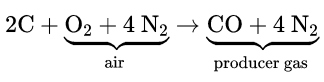
2 . From carbon dioxide: When CO2 is passed over red hot carbon, zinc, Iron etc., it is reduced to CO.
CO2 + C→ 2CO; CO2 + Zn→CO + ZnO
CO2 + Fe→FeO + CO
3. From metal oxides: Carbon reduces die oxides of zinc, lead or iron to produce CO.
ZnO + C→Zn + CO; Fe2O3 + 3C →2Fe + 3CO
4. From nickel tetracarbonyl: Pure CO is obtained when nickel tetracarbonyl vapour is heated above 150°C
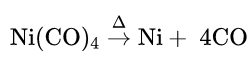
Carbon Monoxide’s Physical properties
- Carbon monoxide is a colourless, tasteless, odourless gas which is lighter than air. © It is slightly soluble in water.
- It is a neutral oxide.
- It is a highly poisonous gas. If only a volume of CO is present in 10,000 volumes of air, then that air is considered to be poisonous.
- Carbon monoxide molecule
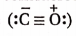 with a lone pair of electrons on carbon combines with Fe-atom present in haemoglobin of the blood to form a very stable complex compound named carboxyhaemoglobin.
with a lone pair of electrons on carbon combines with Fe-atom present in haemoglobin of the blood to form a very stable complex compound named carboxyhaemoglobin.
Hb + CO → HbCO; (Hb =Haemoglobin) As CO is almost 100 times more rigidly bonded to Fe-atom than O2, O2 can no longer combine with haemoglobin.
In other words, haemoglobin fails to act as an oxygen-carrier. As a consequence, the body tissues become slackened due to lack of oxygen and ultimately causing death In case of CO poisoning, the patient should immediately be taken to an open area and artificial respiration with carbogen (a mixture of oxygen and 5-10% CO2) should be started.
Carbon Monoxide Chemical properties
1. Combustion:
Carbon monoxide is itself a combustible gas but does not support combustion. It burns in the air with a blue flame and is oxidised to C02. Because of evolution of a large amount ofheat, CO is used as fuel.
2CO + O2 → 2CO2 + 135.2 kcal
The two important fuels containing carbon monoxide are water gas and producer gas. Water gas contains 50% of H2, 40% of CO, 5% of CO2 and 5% of CH4 and N2 while producer gas contains 25% of CO, 4% of CO2,70% of N2 and traces of H2, CH4 and O2
2. Reducing property:
Carbon monoxide is a powerful reducing agent. The oxidation number of carbonin CO is +2 and the highest oxidation number of carbon is +4. So, CO tends to be oxidised and behaves as a strong reducing agent. Various metal oxides are reduced by CO to the corresponding metal.
CuO + CO→Cu + CO2 ; PbO + CO→Pb + CP
ZnO + CO→Zn + CO2; Fe2O3 + 3CO→2Fe + 3CO2
At 90°C, CO reduces iodine pentoxide (12Os) to give violet-coloured iodine. This reaction is called the Ditte reaction.
I2O5+ 5CO→I2 + 5CO2
3. Reaction with sodium hydroxide:
Being a neutral oxide CO does not react with alkali or base under ordinary conditions. But at 200°C and under high pressure, it reacts with caustic soda solution to yield sodium formate.
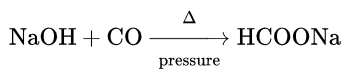
4. Absorption of CO:
When CO is passed through an ammoniacal or acidified cuprous chloride solution, it gets absorbed in that solution to give a white crystalline addition compound as a precipitate. CO can be separated from a gas mixture by this process.
Cu2Cl2 + 2CO + 4H2O→ 2[CuClCOH2O]↓
The addition compound evolves CO on heating.
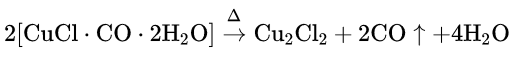
5. Formation of addition compounds:
1. In the presence of sunlight, CO combines directly with chlorine gas to form carbonyl chloride or phosgene gas. It is a colourless poisonous gas:
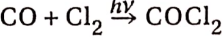
2. CO reacts with sulphur vapour to produce carbonyl sulphide.
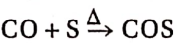
3. CO combines with many transition metals to form metal carbonyl compounds. For example, CO reacts with nickel powder at 30-40°C under ordinary pressure to form nickel tetracarbonyl. Again, at 200°C and 100 atmosphere pressure, CO reacts with freshly reduced iron to form pentacarbonyl.
Ni + 4CO →Ni(CO)4; Fe + 5CO→ Fe(CO)5
6. Formation of organic compounds:
Hydrogen reacts with CO at 350°C in the presence of Ni or Pt catalyst to yield methane. If the reaction is carried out at 300°C and 200 atmospheric pressure in the presence of ZnO and Cr2O3 catalyst, methyl alcohol is produced. The oxidation number of carbon in CO decreases from +2 to -4 in methane and to -2 in methyl alcohol.
Therefore, in these two cases, CO exhibits its oxidising property.
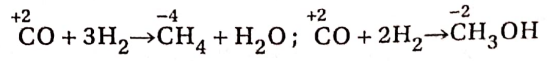
Identification of carbon monoxide
1. Carbon monoxide burns in air with a blue flame and the gaseous product turns lime water milky [H2 also burns with a blue flame but in this case, steam is formed which turns white anhydrous copper sulphate blue.
2. CO is completely absorbed by the Cu2Cl2 solution in a cone. hydrochloric acid or ammonium hydroxide and as a result, a white crystalline addition compound is precipitated.
3. When a filter paper soaked with a solution of platinum or palladium chloride is held in CO gas, the paper turns pink-green or black due to the reduction ofthe metal salts.
PtCl2 + CO + H2O→ Pt (pink-green) + CO2 + 2HCl
PdCl2 + CO + H2O→Pd (black)+ CO2 + 2HCl
4. When CO gas is passed through an ammoniacal AgNO3 solution, the solution becomes brown
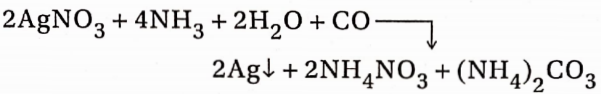
5. When a dilute solution of blood shaken with CO, is subjected to spectroscopic analysis, the observed band in the spectrum indicates the presence of CO. The presence of traces ofCOin air can be detected by this experiment.
6. The presence of a very small amount of CO in the air can be detected with the help of halamite tube or colour detector tube. When air containing CO is introduced into this tube I2O5 present in the tube reacts with CO to liberate I2
Because of the violet colour of evolved I2, the colour of the tube changes and the presence of CO in the air is indicated
I2O2 + 5CO→ I2 (Ditte reaction) + 5CO2
Structure of carbon monoxide
Both the carbon and the oxygen atoms in a CO molecule are sp -hybridised. One of |> the sp -hybrid orbital of each atom is used to form a C —O cr -bond while the other sp -orbital of each contains a lone pair of electrons. The two unhybridised 2p -orbitals of each atom are involved in the formation of two pn-pn bonds. In terms of resonance, the CO molecule can be best represented as a resonance hybrid of the following two i resonance structures(I and II).
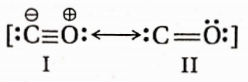
The resonance structure (I) is relatively more stable because of the fulfilment of the octet of both atoms.
Uses Of carbon monoxide:
- CO is used as fuel in the form of producer gas or water gas.
- It is used as a reducing agent in the extraction of metals.
- It is used for the preparation of pure nickel by Mond’s process.
- It is used for the
- Preparation of methanol, methane, formic acid and synthetic petrol (Fischer-Tropsch process).
Preparation of pure nickel:
Ni(CO)4 is prepared by the reaction between impure nickel and carbon monoxide. Ni(CO)4 is then allowed to decompose by heating to 1.50°C to get pure nickel.
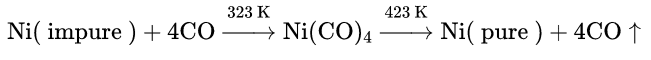
2. Carbon dioxide
Carbon dioxide Laboratory preparation:
At ordinary temperature, CO2 is prepared in the laboratory by the action of diluting HCl on calcium carbonate (CaCO3) or marble.
CaCO3 + 2HCl → CaCl2 + CO2 ↑ + H2O
The gas is collected in the gas jar by the upward displacement of air, as it is 1.5 times heavier than air. Carbon dioxide thus produced contains a small amount of HC1 and water vapour. The gas is then passed successively through NaHCO3 solution and cone, sulphuric acid to remove HCl vapour and water vapour respectively.
Dilute sulphuric acid cannot be used for the preparation of CO2 from marble or limestone:
This is because sulphuric acid reacts with CaCO3 to produce insoluble; CaSO4 which forms a layer of CaCO3. This insoluble layer prevents CaCO3 from reacting with the acid and as a result, the evolution of CO2 ceases within a very short time
CaCO3 + H2SO4 →CaSO4+ CO2 + H2O
On the other hand, when dilute hydrochloric acid is, used, highly soluble calcium chloride (CaCl2) is formed. So, the reaction proceeds without any interruption
CO2 can be prepared by the action of dilute H2SO4 on Na2CO3:
The salt, Na2SO4 produced soluble in water or dilute H2SO4
Na2CO3 + H2SO4→ Na2 SO4 + CO2 + H2O
At ordinary temperatures, CO2 is highly soluble in water. Therefore, it is not collected by the downward displacement of water. The solubility of CO2 in hot water is very low and hence it can be collected over hot
Carbon dioxide Other methods of preparation:
1. From carbonate salts:
Except for alkali metal carbonates, all other carbonates undergo thermal decomposition to produce CO2 and the oxides ofthe corresponding metals.
BaCO3 decomposes only at very high temperatures.
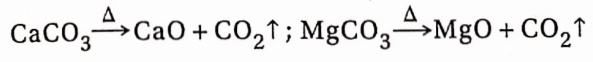
Calcium carbonate or limestone is thermally decomposed (1000°C) for the preparation of carbon dioxide on a commercial scale.
2. From bicarbonate salts:
Bicarbonates of all the elements decompose on heating with the evolution of CO2
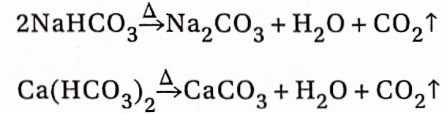
3. From fermentation:
A large amount of C02 is obtained as a by-product during the manufacture of ethyl alcohol by fermentation of sugar

From water gas: Water gas is industrially prepared by passing steam through a bed of white-hot coke at about 100°C.
C + H2 O →CO + H2 When a mixture of water gas and excess of steam is passed over (Fe2O3+ Cr2 O3) catalyst heated at 400°C, CO is oxidised to CO2
(CO + H2) + H2O → CO2 + 2H2
The gaseous product is then passed through a solution of potassium carbonate when C02 is completely absorbed and KHCO3 is formed. H2 and unconverted CO pass out. When the resulting KHC03 solution is boiled, CO2 is obtained.
K2CO3 + CO2 + H2O→ 2KHCO3
Carbon dioxide Physical properties
- Carbon dioxide is a colourless, odourless and tasteless gas having slightly acidic properties.
- CO2 is 1.5 times heavier than air. So, this gas often accumulates in abandoned wells or pits and because of this, severe breathing problems are caused in such places.
- By the application of pressure (nearly 40 atmospheric pressure and a temperature < 40°C), CO2 can be easily liquefied.
- When liquid CO2 is allowed to vaporise rapidly by releasing the pressure, it further gets cooled down and freezes like ice. This is called dry ice or cardice.
- When solid carbon dioxide is allowed to evaporate at atmospheric pressure, it gets converted into the vapour state without passing through the intermediate liquid state. Therefore, unlike ordinary ice, it does not wet the surface of the substance and because of this, it is called dry ice.
- It is highly soluble in water (1.7 cm³ of CO2 dissolves in 1 cm³ of water). The solubility increases with an increase in pressure. Aerated waters such as soda water, lemonade etc. contain CO2 under pressure.
- When the cork of the bottle of aerated water is opened, the pressure is released and excess CO2 escapes in the form of bubbles. Its solubilityin water, however, decreases with temperature rise.
Carbon dioxide Chemical properties
1. Combustion:
Carbon dioxide is neither combustible nor helps in combustion. When it (heavier than air) falls on a binning substance, it removes air from the surface of the substance and thereby the substance can no longer remain in contact with air. As a result, the fire is extinguished. A burning jute stick when inserted into a jar of CO2, extinguishes.
However, when a burning Mgribbon or metallic sodium is inserted into a CO2 jar, it continues to bum with the separation of black carbon.
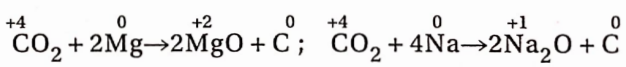
During the burning of such metals, the temperature, due to the liberation of a large amount of heat, is so high that CO2 decomposes into carbon and O2 and it is the oxygen which helps in the burning ofthe metals.
In these reactions, CO2 acts as an oxidising agent and itself gets reduced to carbon. These reactions prove the existence of carbon in CO2 It is to be noted that the oxidation number of carbon in CO2 is +4 and this is its highest state of oxidation.
Thus, there is no possibility of an increase in its oxidation number, i.e., CO2 cannot be further oxidised. That is why CO2 cannot exhibit any reducing property. For the same basic reason, CO2 is not combustible [CO, on the other hand, is combustible because in this case, the oxidation number of carbon may increase from +2 to +4 ].
2. Acidic property:
Carbon dioxide is an acidic oxide. It dissolves in water forming an unstable dibasic acid called carbonic acid (H2CO3). CO2 is, therefore, regarded as the anhydride of carbonic acid.
H2CO3 is known only in solution and when the solution is heated, CO2 is evolved out The solution turns blue litmus red but it cannot change the colour of methyl orange. H2CO3 forms two types of salts, bicarbonates (HCO3 ) and carbonates (CO32-). Being an acidic oxide, CO2 combines directly with strongly basic oxides such as CaO, Na2O etc. to form their corresponding salts.
CaO + CO2→ CaCO2; Na2O + CO2 →Na2CO3
Reaction with alkali:
When CO2 is passed through a strong alkaline solution of NaOH, a carbonate salt is first formed. If the passage of CO2 is continued for a long time, white crystals of sparingly soluble sodium bicarbonate are precipitated. The bicarbonate salt decomposes on heating to form carbonate salt, CO2and water.
2NaOH + CO2→ Na2 CO3+ H2O
Na2CO3 + CO2+ H2O →2NaHCO3
Rection with lime water:
When CO2 is passed through lime water, the solution becomes milky due to the formation of white insoluble calcium carbonate. However, when an excess of CO2 gas is passed through this milky solution, its milkiness disappears as insoluble calcium carbonate gets converted into soluble calcium bicarbonate
Ca(OH)2 + CO2 →CaCO3.↓ (white) +H2O
CaCO3 + CO2 + H2O→Ca(HCO3)O2 (soluble)
On heating, calcium bicarbonate decomposes to form calcium carbonate, CO2 and water and as a result, the clear solution becomes milky again.
Ca(HCO3)2→CaCO3↓ + CO2 + H2O
3. Manufacture of sodium carbonate:
When CO2 gas is passed through a concentrated solution of sodium chloride (brine) saturated with ammonia at 30-40°C, white crystals of sodium bicarbonate are precipitated. The reaction occurs in two stages
NH3 + CO2 + H2O ⇌ NH4HCO3
NH4HCO3 + NaCI→NaHCO3↓+ NH4Cl
Sodium carbonate is prepared by thermal decomposition of sodium bicarbonate. The Solvay process for the manufacture of sodium carbonate is based on this reaction.
4. Production of ammonium sulphate:
This is carried out by passing CO, and NH3 gases through a slurry of powdered gypsum (CaSO4, 2H2O) in water. At first, NH3 and CO2 react together in the presence of water to form ammonium carbonate. It then reacts with calcium sulphate (gypsum) to form calcium carbonate and ammonium sulphate by double decomposition.
2NH3 + CO2 + H2O ⇌ (NH4)2CO3
CaSO4 + (NH4)2CO3→ CaCO3 ↓+ (NH4)2SO4
The nitrogenous fertiliser ammonium sulphate is manufactured by using this reaction. In this process, (NH4)2SO4 is produced without using H2SO4.
5. Production of urea:
At 200-210°C and 150 atm pressure, C02 reacts with ammonia to produce urea.
CO2 + 2NH3 ⇌ NH4COONH2 (Ammonium carbamate) ⇌ CO(NH2)2 (Urea) + HO2
The important fertiliser, urea is manufactured on a large scale by using this reaction.
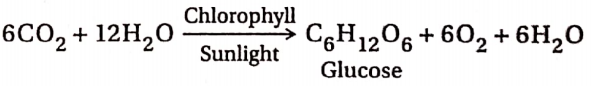
6. Photosynthesis:
Plants absorb atmospheric carbon dioxide. In the presence of chlorophyll and sunlight, the absorbed CO2 combines with water (absorbed from the soil) to form glucose, water and oxygen. This process is called photosynthesis. In this process, CO2 is reduced to carbohydrates by water
7. Reduction of CO2: When CO2 is passed over heated C, Fe, Zn etc., it is reduced to CO

Identification of carbon dioxide
- It extinguishes a burning stick.
- Lime water becomes turbid when CO2 is passed through it. When excess of CO2 is passed through it, the turbidity disappears but when that clear solution is boiled, the turbidity reappears.
- N2 gas also extinguishes burning sticks but it does not turn the water milky. Again, SO2 gas also turns lime water milky but unlike CO2 it reacts with an acidified solution potassium dichromate and changes the colour of the solution from orange to green
Uses Of carbon dioxide
- CO2 is used in the manufacture of sodium carbonate by the Solvay process and also for the manufacture of fertilisers such as urea, ammonium sulphate etc.
- CO2 is used in fire extinguishers.
- It finds extensive use in the preparation of aerated waters such as soda water, lemonade etc. and baking powder.
- Solid carbon dioxide i.e., dry ice is used as a refrigerant under the commercial name drikold. Dry ice is also used for making cold baths in the laboratory by mixing it with some volatile organic solvents.
- It is extensively used as a coolant for preserving perishable articles in the food industry, for curing local burns and for surgical operations of sores.
Supercritical CO2 :
- Supercritical CO2 is used as a. solvent to extract organic compounds from their natural sources, for example, caffeine from coffee beans, perfumes from flowers etc.
- It is used under the name carbogen (a mixture of 95% O2 and 5% CO2) for the artificial respiration of patients suffering from pneumonia and affected by poisonous gases (CO poisoning).
- Liquid CO2 is used as a substitute for chlorofluorocarbons in aerosol propellants.
Fire extinguisher
It is a specially designed metallic pressure vessel having a nozzle at one end. A glass bottle containing dilute sulphuric acid is placed inside it and the remaining portion of the vessel is filled with concentrated solution of sodium bicarbonate. When required, the glass bottle can be broken by pressing a knob fitted with the vessel at the other end.
When the glass bottle is broken, the add comes in contact with sodium bicarbonate solution and reacts to yield copious CO2 gas. The gas, ejected under high pressure through the nozzle, falls on the burning substance and as a result, the fire gets extinguished
Na2CO3 + H2SO4 →Na2SO4 + CO2 ↑+ H2O

Baking powder
Baking powder which is used Fire extinguisher in the preparation of bread consists of a dry mixture of potassium hydrogen tartrate, NaHCO3, tartaric acid and -starch. When this tithe comes in contact with water present in the bread, a chemical reaction leading to the formation of CO2 occurs.
The resulting CO2 gas evolved in the form of bubbles making the bread porous and soft. Moreover, NaHCO3 and tartaric acid also produce CO2 on thermal decomposition

Structure of carbon dioxide:
In a CO2 molecule, the carbon atom is sp -hybridised whereas the oxygen atoms are sp² – hybridised. Carbon forms two σ -bonds and two pπ- bonds with two oxygen atoms. The shape of the carbon dioxide molecule is, therefore, linear. The molecule is symmetrical (the two bond moments cancel each other) and hence, it is non-polar. The C —O bond length is 1.15Å. CO2 can be represented as a resonance hybrid of the following three structures: