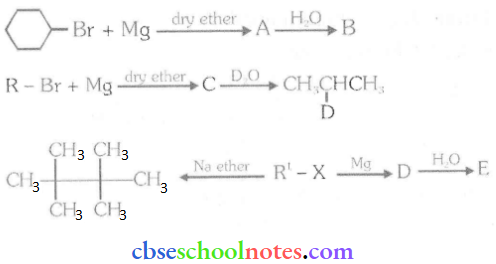
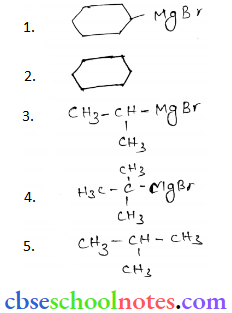
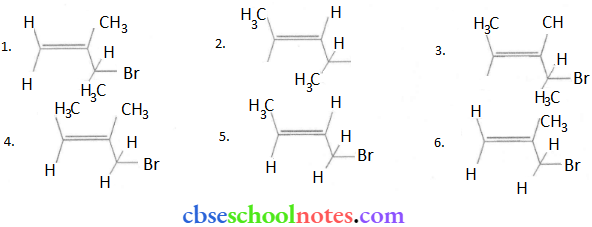
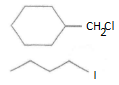
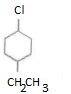
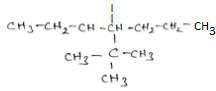
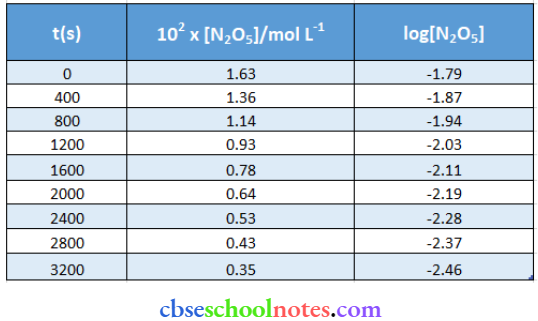
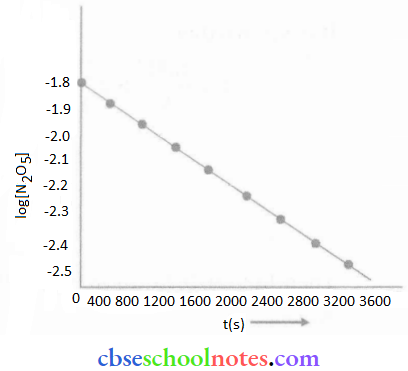
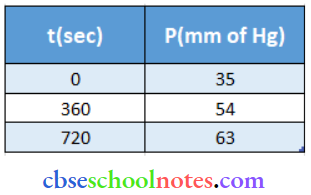
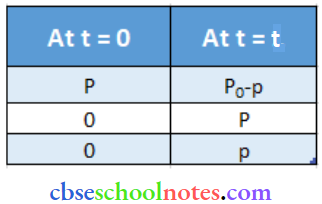
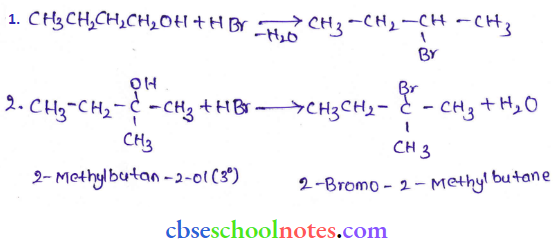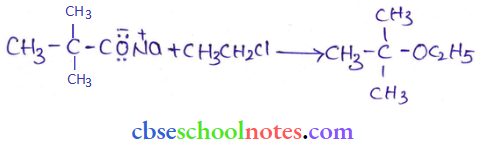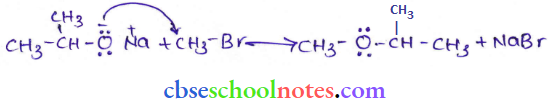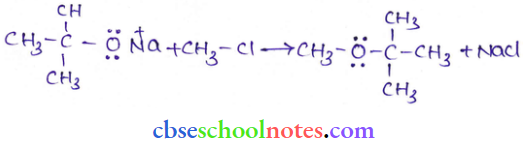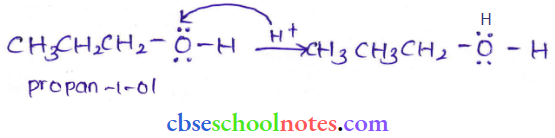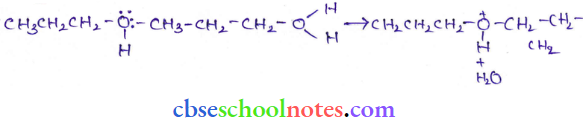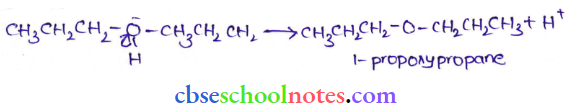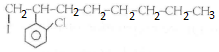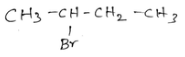Important Questions for Class 12 Chemistry Chapter 1 Solutions
Question 1. Calculate the mass percentage of benzene (C6H6) and carbon tetrachloride (CCI4) if 22g of benzene is dissolved in 122 g of carbon tetrachloride.
Answer:
Total mass = 22 + 1 22 = 144 g
Mass % of benzene = \(\frac{22 \times 100}{144}=15.28\)
Mass 7r of CCI = \(\frac{122}{144} \times 100=84.72\)
Question 2. Calculate the mole fraction of benzene in a solution containing 30% by mass in carbon tetrachloride.
Answer:
Mass of benzene = 30 g
Mass of carbon tetrachloride = 100- 30 = 70g
Molar mass of benzene (C6H6) = 78,
Molar mass of carbon tetrachloride = 154
Moles of benzene = \(\frac{w}{m}=\frac{30}{154}=\) = 0.385
Moles of carbon tetrachloride = \(\frac{w}{m}\)
Total moles = 0.385 + 0.455 = 0.840
⇒ Mole fraction of benzene = \(\frac{\text { moles of benzene }}{\text { total moles }}=\frac{0.385}{0.840}=0.458\)
Mole fraction of carbon tetrachloride = 1 -0.458 = 0.542
Question 3. Calculate the molarity of each of the following solutions:
- 30 g of Co(NO3)2 . 6H2O in 4.3 L of solution.
- 30 mL of 0.5 M H2SO4 diluted to 500 mL.
Answer:
1. Molar mass of Co(NO3)2. 6H2O = 310.7
⇒ Molarity = \(\frac{w}{m \times V(\text { lit })}=\frac{30}{310.7 \times 4.3}=0.022\)
2. M1V1 = M2V2
M1 = 0.5 V1 = 30 mL. M2 = ?. V2 = 500mL
0.5 × 30 = M2 × 500
or M2 = 0.03
Read and Learn More Class 12 Chemistry with Answers Chapter Wise
Question 4. Calculate the mass of urea ( NH2CONH2) required in making 2.5 kg of 0.25 molal aqueous solution.
Answer:
0.25 molal aqueous solution means
Moles of urea = 0.25, Mass of water = 1 kg = 1000 g
0.25 mole urea = 0.25 × 60 = 15 gram
Total mass of solution = 1000+ 15 = 101 5g = 1.015 kg
∵ 1.015 kg solution contains urea = 15 g
∴ 2.5 kg solution contains urea = \(\frac{15 \times 2.5}{1.015}=36.94 \mathrm{gram}\)
Question 5. Calculate
- Molality,
- Molarity,
- Mole fraction of KI if the density of 20% (mass/mass) aqueous KI is 1 .202 g/mL.
Answer:
20% mass/mass aqueous KI means
Mass of KI = 20 g, Mass of water = 80g,
Mass of solution = 100 g
For molality:
Molar mass of KI = 39 + 127 = 166
⇒ \(\text { Molality }=\frac{w}{m \times \text { mass of solvent in } \mathrm{kg}}=\frac{20}{166 \times 80 \times 10^{-3}}=1.51\)
For Molarity:
⇒ \(\text { Volume of solution }=\frac{\text { mass }}{\text { density }}=\frac{100}{1.202}=83.2 \mathrm{ml}\)
⇒ \(\text { Molarity }=\frac{w}{\mathrm{~m} \times \mathrm{V}(\text { lit })}=\frac{20}{166 \times 83.2 \times 10^{-3}}=1.448\)
⇒ \(\text { Moles of } \mathrm{KI}=\frac{\mathrm{w}}{\mathrm{m}}=\frac{20}{166}=0.120\)
⇒ \(\text { Moles of water }=\frac{w}{m}=\frac{80}{18}=4.444\)
Total moles = 0.120 + 4.444 = 4.564
⇒ \(\text { Mole fraction of } \mathrm{KI}=\frac{0.120}{4.564}=0.0263\)

Question 6. H2S, a toxic gas with a rotten egg smell, is used for the qualitative analysis. If the solubility of H2S in water at STP is 0.195 m, calculate Henry’s law constant. Mass of solvent (water) = 1kg = 1000g.
Answer:
Solubility = 0. 195 moles in one kg of water
Mass of solvent (water) = 1 kg = 1000g
⇒ \(\text { Moles of water }=\frac{w}{m}=\frac{1000}{18}=55.55\)
Total moles = 0. 1 95 + 55.55
Mole fraction of H2S(x) in solution \(=\frac{0.195}{0.195+55.55}=0.0035\)
The pressure of H2S at STP = 0.987 bar.
Since partial pressure of the gas is given,
⇒ PH2S = KH × X
or 0.987 = KH × 0.0035
or KH = 282 bar
Question 7. Henry’s law constant for CO2 in water is 1.67 x 108 Pa at 298 K. Calculate the quantity of CO2 in 500 mL of soda water when packed under 2.5 atmospheric CO2 pressure at 298 K.
Answer:
KH = 1.67 x 108Pa
⇒ PCo2 = 2.5 atm = 2.5 x 101325 Pa
⇒ PCo2 = KH × XCo2
or 2.5 x 101325 = 1.67 x 108 × XCo2
or XCo2 = 1.517 x 10-3
⇒ XCo2 = \(\frac{{ }^n \mathrm{CO}_2}{{ }^n \mathrm{H}_2 \mathrm{O}+{ }^n \mathrm{CO}_2}=\frac{{ }^n \mathrm{CO}_2}{{ }^n \mathrm{H}_2 \mathrm{O}}=1.517 \times 10^{-3}\)
nCo2 is neglected in the denominator in comparison to nH2O.
Water = 500 ml, = 500 g,
⇒ \(\text { Moles of water }=\frac{1 \times 8.314 \times 10^3 \times 310}{185000}\)
⇒ \(\frac{{ }^n \mathrm{CO}_2}{{ }^n \mathrm{H}_2 \mathrm{O}}=1.517 \times 10^{-3}\)
or, \(\frac{{ }^n \mathrm{CO}_2}{27.78}=1.517 \times 10^{-3}\)
or nCo2 = 42.14 x 10-3
Mass of CO2 = moles x molar mass
= 42.14 x 10-3 × 44 = 1.854 gram
Question 8. The vapour pressure of pure liquids A and B are 450 and 700 mm Hg respectively at 350 K. Find out the composition of the liquid mixture if the total vapour pressure is 600 mm Hg. Also, find the composition in the vapour phase.
Answer:
P0A = 450 mm. P0B= 700 mm, PTotal = 600 mm
A solution contains two liquids.
Applying Raoulfs law liquids: Ps = P0A XA + P0BXB
or Ps = P0AXA = P0B(1-X) [XA + XB =1]
or Ps = P0B= XA(P0A-P0B)
600=700+XA (450-700)
or, \(X_A=\frac{100}{250}=0.40\)
⇒ XB = 1-XA = 1- 0.40 = 0.60
⇒ PA = P0AXA = 450 × 0.40 = 180 mm
⇒ PB = P0BXB = 700 × 0.60 = 420 mm
Total vapour pressure = 180 + 420 = 600 mm
Mole fraction of A in vapour phase = \(\frac{P_A}{P_{\text {total }}}=\frac{180}{600}=0.30\)
Mole fraction of B in vapour phase = \(\frac{P_B}{P_{\text {total }}}=\frac{420}{600}=0.70\)
Question 9. The vapour pressure of pure water at 298 K is 23.8 mm Hg. 50 grams of urea (NH2CONH2) is dissolved in 850 g of water. Calculate the vapour pressure of water for this solution and its relative lowering.
Answer:
A solution contains a non-volatile solid. Our aim is to calculate Ps and \(\frac{P_A^o-P_S}{P_S}=\frac{W \times M}{m \times W}\)
P0A = 23.8 w = 50.. W = 850. Ps = ?. M = 18 for H2O, m= 60 for urea
⇒ \(\frac{\mathrm{P}_{\mathrm{A}}^o-\mathrm{P}_{\mathrm{S}}}{\mathrm{P}_{\mathrm{S}}}=\frac{\mathrm{w} \times \mathrm{M}}{\mathrm{m} \times \mathrm{W}}\)
or \(\frac{23.8-P_S}{P_S}=\frac{50 \times 18}{60 \times 850}=\frac{3}{170}\)
or Ps = 23.387
Again, relative lowering in V.P.
⇒ \(\frac{\mathrm{P}_{\mathrm{A}}^0-\mathrm{P}_{\mathrm{S}}}{\mathrm{P}_{\mathrm{A}}^0}=\frac{23.8-23.387}{23.8}=0.017\)
Question 10. The boiling point of water at 750 mm is 96.63°C. How much sucrose is to be added to 500 g of water such that it boils at 100°C? K = 0.52 K kg/mol.
Answer:
ΔTb = 100- 96.63 = 3.37
w = ?, W = 500 g, M = 342 for sucrose
Kb = 0.52 K kg/mol
⇒ \(\Delta \mathrm{T}_{\mathrm{b}}=\frac{\mathrm{K}_{\mathrm{b}} \times \mathrm{W} \times 1000}{\mathrm{M} \times \mathrm{W}}\)
or \(3.37=\frac{0.52 \times w \times 1000}{342 \times 500}\)
or w= 1108.2 g
Question 11. Calculate the mass of ascorbic acid (vitamin C, C6H8O6) to be dissolved in 75g acetic acid to lower its melting point by 1.5°C. Kf = 3.9 K kg/mol.
Answer:
⇒ \(\Delta \mathrm{T}_f=\frac{\mathrm{K}_{\mathrm{f}} \times \mathrm{w} \times 1000}{\mathrm{M} \times \mathrm{W}}\)
The formula for lowering in freezing point or melting point is the same.
ΔTf = 1.5°, w = ?. W = 75g
M for ascorbic acid = 176
⇒ \(1.5=\frac{3.9 \times w \times 1000}{176 \times 75}\)
or w= 5.08 g
Question 12. Calculate the osmotic pressure in pascals excited by a solution prepared by dissolving 1.0 g of polymer of molar mass 185000 in 450 mL of water at 37°C.
Answer:
Since mass of solute is given, πV = \(\frac{w R T}{m}\)
π = ?, V = 0.450 litre, w = Ig, m = 185000,
R = 8.314 kPa LK-1 mol-1
= 8.314 kPa LK-1 mol-1
⇒ \(\pi \times 0.450=\frac{1 \times 8.314 \times 10^3 \times 310}{185000}\)
or π = 30.96 Pa
Question 13. Define the term solution. How many types of solutions are formed? Write briefly about each type with an example.
Answer:
Homogeneous mixtures of two or more than two components are known as solutions. There are three types of solutions.
- Gaseous solution: The solution in which the solvent is a gas is called a gaseous solution. In these solutions, the solute may be liquid, solid, or gas. For example, a mixture of oxygen and nitrogen gas is a gaseous solution.
- Liquid solution: The solution in which the solvent is a liquid is known as a liquid solution. The solute in these solutions may be gas. liquid, or solid. For example, a solution of ethanol in water is a liquid solution.
- Solid solution: The solution in which the solvent is a solid is known as a solid solution. The solute may be gas, liquid or solid. For example, a solution of copper in gold is a solid solution.
Question 14. Give an example of a solid solution in which the solute is a gas.
Answer: A solution of hydrogen in palladium is a solid solution in which the solute is a gas.
Question 15. Define the following terms:
- Mole fraction
- Molality
- Molarity
- Mass percentage
Answer:
Mole fraction: The mole fraction of a component in a mixture is defined as the ratio of the number of moles of the component to the total number of moles of all the components in the mixture.
i.e. Mole fraction of a component \(=\frac{\text { Number of moles of the component }}{\text { Total number of moles of all components }}\)
The mole fraction is denoted by ‘X’.
If in a binary solution, the number of moles of the solute and the solvent are n and n respectively, then the mole fraction of the solute in the solution is given by.
⇒ \(x_A=\frac{n_A}{n_A+n_B}\)
Similarly, the mole fraction of the solvent in the solution is given as:
⇒ \(x_B=\frac{n_A}{n_A+n_B}\)
Molality: Molality (m) is defined as the number of moles of the solute per kilogram of the solvent. It is expressed as:
⇒ \(\text { Molality }(m)=\frac{\text { Moles of solute }}{\text { Mass of solvent in } \mathrm{kg}}\)
Molarity: Molarity (M) is defined as the number of moles of the solute dissolved in
one Litre of solution. It is expressed as:
⇒ \(\text { Molarity }(M)=\frac{\text { Moles of solute }}{\text { Volume of solvent in Litre }}\)
Mass percentage: The mass percentage of a component of a solution is defined as the mass of the solute in grams present in l00g of the solution. It is expressed as:
Mass % of a component \(=\frac{\text { Mass of component in solution }}{\text { Total mass of solution }} \times 100\)
Question 16. Concentrated nitric acid used in laboratory work is 68% nitric acid by mass in aqueous solution. What should be the molarity of such a sample of the acid if the density of the solution is 1.504 g mL-1?
Answer:
Concentrated nitric acid used in laboratory work is 68% nitric acid bv mass in an aqueous solution. This means that 68 g of nitric acid is dissolved in 100 g of the solution.
Molar mass of nitric acid (HNO3) = 1 × 1 + 14 + 3 × 16 = 63 g mol-1
Then, number of moles of HNO3 = \(\frac{68}{6.3} \mathrm{~mol}=1.079 \mathrm{~mol}\)
Given, Density of solution = 1.504 g mL-1
∴ Volume of 100 g solution = \(\frac{100}{1.504} \mathrm{~mL}\) = 66.49 mL = 66.49 x 10-3
Molarity of solution = \(\frac{1.079}{66.49 \times 10^{-3} \mathrm{~L}}=16.23 \mathrm{M}\)
Question 17. A solution of glucose in water is labelled as 10% (w/w), what would be the molality and mole fraction of each component in the solution? If the density of the Solution is 1.2 g mL-1, then what shall
be the molarity of the solution?
Answer:
10% w/w solution of glucose in water means that 10 g of glucose is present in lOOg of the solution i.e., 10 g of glucose is present in ( 100- 1 0) g = 90 g of water.
Molar mass of glucose (C6H12O6) = 6 × 12 + 12 × 1 + 6 × 16 = 180 g mol-1
Then, number of moles of glucose = \(\frac{10}{180} \mathrm{~mol}=0.056 \mathrm{~mol}\)
∴ Molality of solution = \(\frac{0.056}{0.09 \mathrm{~kg}}=0.62 \mathrm{~m}\)
Number of moles of water = \(\frac{90 \mathrm{~g}}{18 \mathrm{gmol}^{-1}}=5 \mathrm{~mol}\)
⇒ Mole fraction of glucose (xg) = \(\frac{0.056}{0.056+5}=0.011\)
And, mole fraction of water xw = 1-Xg = 1- 0.011 = 0.989
If the density of the solution is 1.2 g mL-1. then the volume of the 100 g solution can be given as:
⇒ \(\mathrm{V}=\frac{100 \mathrm{~g}}{1.2 \mathrm{~g} \mathrm{~mL}^{-1}}=83.33 \mathrm{~mL}=83.33 \times 10^{-3} \mathrm{~L}\)
Molarity of the solution = \(\frac{0.056 \mathrm{~mol}}{83.33 \times 10^{-3} \mathrm{~L}}=0.67 \mathrm{M}\)
Question 18. How many mL of 0.1 M HCl are required to react completely with a 1 g mixture of Na2CO3 and NaHCO3. containing equimolar amounts of both?
Answer:
Let the amount of Na2CO3. in the mixture be x g.
Then, the amount of NaHCO3. in the mixture is (1 – x) g,
The molar mass of Na2CO3 = 2 × 23 +1 × 12 + 3 × 16= 106 g mol-1
Number of moles Na2CO3 = \(\frac{x}{106} \mathrm{~mol}\)
Molar mass of NaHCO3 = 1 x 23 + 1 x 1 x 12 + 3 x 16 = 84g mol-1
Number of moles NaHCO3 = \(\frac{1-x}{84} \mathrm{~mol}\)
According to the question. \(\frac{x}{106}=\frac{1-x}{84}\)
⇒ 84 x = 106 – 106x
⇒ 190x = 106
⇒ x = 0.0053
Therefore, number of moles of Na2CO3 = \(\frac{0.5579}{106} \mathrm{~mol}=0.0053 \mathrm{~mol}\)
And. number of moles of NaHCO3 = \(\frac{1-0.5579}{84}=0.0053 \mathrm{~mol}\)
HCl reacts with Na2CO3 and NaHCO3 according to the following equation.
⇒ \(\underset{2 \mathrm{~mol}}{2 \mathrm{HCl}}+\underset{\text { Imol }}{\mathrm{Na}_2 \mathrm{CO}_3 \longrightarrow 2 \mathrm{NaCl}}+\mathrm{H}_2 \mathrm{O}+\mathrm{CO}_2\)
⇒ \(\underset{\text { 1 mol }}{\mathrm{HCl}}+\underset{\text { 1 mol }}{\mathrm{NaHCO}_3} \longrightarrow \mathrm{NaCl}+\mathrm{H}_2 \mathrm{O}+\mathrm{CO}_2\)
1 mol of Na2CO3 reacts with 2 mol of HCl
Therefore. 0.0053 mol of Na2CO3 reacts with HCl = 2 × 0.0053 mol = 0.0106 mol.
Similarly. 1 mol of NaHCO3 reacts with I mol of HCl.
Therefore. 0.0053 mol of NaHCO reacts with 0.0053 mol of HCl.
Total moles of HCI required = (0.0106 + 0.0053) mol =0.0159 mol
0.1 mol of HCl is preset in 1000 ml. of the solution.
Therefore 0.0159 mol of HCI is present in the solution
⇒ \(\frac{1000 \times 0.0159}{0.1} \mathrm{~mol}=159 \mathrm{~mL}\)
Hence, 159 mL of 0.1 M of HCl is required to react completely with 1 mixture of NaCO3 and NaHCO3. containing equimolar amounts of both.
Question 19. A solution is obtained by mixing 300 g of 259f solution and 400 g of 40% solution by mass. Calculate the mass percentage of the resulting solution.
Answer: The total amount of solute present in the mixture is given by
⇒ \(300 \times \frac{25}{100}+400 \times \frac{40}{100}=75+160=235 \mathrm{~g}\)
Total amount of solution = 300 + 400 = 700 g
Therefore, the mass percentage (w/w) of the solute in the resulting solution
⇒ \(\frac{235}{700} \times 100 \%=33.57 \%\)
And, mass percentage (w/w) of the solvent in the resulting solution.
= (100-33.57%) = 66.43%
Question 20. An antifreeze solution is prepared from 222.6 g of ethylene glycol (C2H6O2) and 200 g of water. Calculate the molality of the solution. If the density of the solution is 1.072 g mL-1, then what shall be the molarity of the solution?
Answer:
Molar mass of ethylene glycol (C2H4 (OH)2 = 2 × 12 + 6 × 1 + 2 × 16 = 62 gmo-1
Number of moles of ethylene glycol = \(\frac{222.6 \mathrm{~g}}{62 \mathrm{gmol}^{-1}}=3.59 \mathrm{~mol}\)
Therefore, molality of the solution = \(\frac{3.59 \mathrm{~mol}}{0.200 \mathrm{~kg}}=17.95 \mathrm{~m}\)
Total mass of the solution = (222,6 + 200) g = 422.6 g
Given,
Density of the solution = 1.072 g mL-1
∴ Volume of the solution = \(\frac{422.6 \mathrm{~g}}{1.072 \mathrm{~g} \mathrm{~mL}^{-1}}=394.22 \mathrm{~mL}=0.3942 \times 10^{-3} \mathrm{~L}\)
⇒ Molarity of the solution = \(\frac{3.59 \mathrm{~mol}}{0.39422 \times 10^{-3} \mathrm{~L}}=9.11 \mathrm{M}\)
Question 21. A sample of drinking water was found to be severely contaminated with chloroform (CHCIJ supposed to be a carcinogen. The level of contamination was 15 ppm (by mass):
- Express this in percent by mass
- Determine the molality of chloroform in the water sample.
Answer:
15 ppm (by mass) means 15 parts per million ( 106) of the solution
Therefore, percent by mass = \(\frac{15}{10^6} \times 100 \%=15 \times 10-4 \%\)
Molar mass of chloroform (CHCl3) = 1 × 12 + 1 × 1 + 3 × 35.5 = 119.5 g mol-1
Now. according to the question.
15 g of chloroform is present in 106 g of the solution.
i.e., 15 g chloroform is present in ( 106– 15) ≈ 106g of water
∴ Molality of the solution = \(\frac{\frac{15}{119.5} \mathrm{~mol}}{10^6 \times 10^{-3} \mathrm{~kg}}=1.26 \times 10^{-4} \mathrm{~m}\)
Question 22. What role does the molecular interaction play in a solution of alcohol and water?
Answer:
- In pure alcohol and water, the molecules are held tightly by a strong hydrogen bonding. The interaction between the molecules of alcohol and water is weaker than alcohol-alcohol and water-water interactions.
- As a result, when alcohol and water are mixed, the intermolecular interactions become weaker and the molecules can easily escape.
- This increases the vapour pressure of the solution, which in turn lowers the boiling point of the resulting solution.
Question 23. Why do gases always tend to be less soluble in liquids as the temperature is raised?
Answer:
The solubility of gases in liquids decreases with an increase in temperature. This is because dissolution of gases in liquids is an exothermic process.
Gas + Liquid → Solution + Heat
Therefore, when the temperature is increased, heat is supplied and the equilibrium shifts backwards, thereby decreasing the solubility of gases.
Question 24. State Henry’s law and mention some important applications.
Answer: Henry’s Law states that the partial pressure of a gas in the vapour phase is proportional to the mole fraction of the gas in the solution. If p is the partial pressure of the gas in the vapour phase and x is the mole fraction of the gas, then Henry’s law’ can be expressed as:
p = KHX
where KH is Henry’s law constant
Some important applications of Henry’s law are mentioned below.
- Bottles are sealed under high pressure to increase the solubility of CO2 in soft drinks and soda water.
- Henry’s Jaw states that the solubility of gases increases with an increase in pressure, Therefore, when a scuba diverts deep into the sea, the increased sea pressure causes the nitrogen present in the air to dissolve in his blood in great amounts.
- As a result, when he comes back to the surface, the solubility of nitrogen again decreases and the dissolved gas is released, leading to the formation of nitrogen bubbles in the blood.
- This results in the blockage of capillaries and leads to a medical condition known as ’bends’ or ’decompression sickness’.
- Hence, the oxygen tanks used by scuba divers are filled with air and diluted with helium to avoid bends. The concentration of oxygen is low in the blood and tissue of people living at high altitudes such as climbers.
- This is because, at high altitudes, the partial pressure of oxygen is less than that at ground level. Low-blood oxygen causes climbers to become weak and prevents them from thinking clearly. These are symptoms of anoxia.
Question 25. The partial pressure of ethane over a solution containing 6.56 x 10-1 g of ethane is 1 bar. If the solution contains 5.00 x 10-2 g of ethane, then what shall be the partial pressure of the gas?
Answer: Molar mass of ethane (C2H6) = 2 × 12+ 6 × 1 = 30 g mol-1
∴ Number of moles present in 6.56 x 10-3 g pf ethane = \(\frac{6.56 \times 10^{-3}}{30}=0.218 \times 10^{-3} \mathrm{~mol}\)
Let the number of moles of the solvent be x.
According to Henry’s law, p = KHx
⇒ \(\text { 1bar }=K_H \cdot \frac{0.218 \times 10^{-3}}{0.218 \times 10^{-3}+x}\)
⇒ \(1 \text { bar }=K_H \frac{0.218 \times 10^{-3}}{x}\left(\text { Since } x>>0.218 \times 10^{-3}\right)\)
⇒ \(K_{11}=\frac{x}{0.218 \times 10^{-3}} \text { bar }\)
Number of moles present in 5.00 × 10-2 g of ethane \(\frac{5.00 \times 10^{-2}}{30} \mathrm{~mol}=1.67 \times 10^{-3} \mathrm{~mol}\)
According to Henry’s law, p = KHx
⇒ \(\frac{x}{0.218 \times 10^{-3}} \times \frac{1.67 \times 10^{-3}}{\left(1.67 \times 10^{-3}\right)+x}\)
⇒ \(\frac{x}{0.218 \times 10^{-1}} \times \frac{1.67 \times 10^{-3}}{x}\) (Since, x >> 1 .67 x 10-3)
= 0.764 bar
Hence, the partial pressure of the gas shall be 7.66 bar
Question 26. What is meant by positive and negative deviations from Raoult’s law and how is the sign of ΔsolH related to positive and negative deviations from Raoult’s law?
Answer:
According to Raoult’s law. the partial vapour pressure of each volatile component in any solution is directly proportional to its mole fraction. The solutions which obey Raoult’s law over the entire range of concentration are known as ideal solutions.
- The solutions that do not obey Raoult’s law (non-ideal solutions) have vapour pressure either higher or lower than that predicted by Raoult’s law.
- If the vapour pressure is higher, then the solution is said to exhibit positive deviation, and if it is lower, then the solution is said to exhibit negative deviation from Raoult’s law.
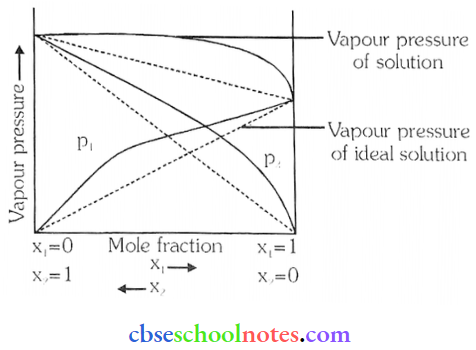
Vapour pressure of a two-component solution showing positive deviation from Raoult’s law.

Vapour pressure of a two-component solution showing negative deviation from Raoult’s law
In the case of an ideal solution, the enthalpy of the mixing of the pure components for forming the solutions is zero.
AsolH = 0
In the case of solutions showing positive deviations, absorption of heat takes place,
AsolH = Positive
In the case of solutions showing negative deviations, the evolution of heat takes place.
AsolH = Negative
Question 27. An aqueous solution of 2% non-volatile solute exerts a pressure of 1.004 bar at the normal boiling point of the solvent. What is the molar mass of the solute?
Answer:
Here, Vapour pressure of the solution at normal boiling point ( p1) = 1.004 bar
Vapour pressure of pure water at normal boiling point ( P01 ) = 1.01 3 bar
Mass of solute, (w2) = 2g
Mass of solvent (water), (w1) = 98 g
Molar mass of solvent (water), ( M1) = 18 g mol-1
According to Raoult’s law,
⇒ \(\frac{p_1^0-p_1}{p_1^0}=\frac{w_2 \times M_1}{M_2 \times w_1}\)
⇒ \(\frac{1.013-1.004}{1.013}=\frac{2 \times 18}{\mathrm{M}_2 \times 98}\)
⇒ \(\frac{0.009}{1.013}=\frac{2 \times 18}{\mathrm{M}_2 \times 98}\)
⇒ \(\mathrm{M}_2=\frac{1.013 \times 2 \times 18}{0.009 \times 98}\) = 41.35g mol-1
Hence, (lie molar mass of the solute is 41.35 g mol-1
Question 28. Heptane and octane form an ideal solution. At 373 K. the vapour pressure of the two liquid components are 105.2 kPa and 46.8 kPa respectively. What will be the vapour pressure of a mixture of 26.0 g of heptane and 35 g of octane?
Answer:
Vapour pressure of heptane (P01) = 105.2 kPa
Vapour pressure of octane (P02) = 46.8 kPa
We know that,
Molar mas of heptane (C7H16 ) = 7 x 12 + 16 x 1 = 100 g mol-1
∴ Number of moles of heptane = \(\frac{26}{100}=0.26 \mathrm{~mol}\)
∴ Number of moles of octane = \(\frac{35}{114} \mathrm{~mol}=0.31 \mathrm{~mol}\)
Mole fraction of heptane, x1 = \(\frac{0.26}{0.26+0.31}=0.456\)
And, mole fraction of octane, x2 = 1 – 0.456 = 0.544
Now, partial pressure of heptane, p1 = x1P01 = 0.456 x 105.2 = 47.97 kPa
Partial pressure of octane, p2 = x2p02 = 0.574 x 46.8 = 26.86 kPa
Hence, the vapour pressure of a solution. PTotal = p1 + p2 = 47.97 + 26.86 = 74.83 kPa
Question 29. The vapour pressure of water is 12.3 kPa at 300 K. Calculate the vapour pressure of 1 molal solution of a non-volatile solute in it.
Answer:
1 molal solution means 1 mol of the solute is present in 1000 g of the solvent (water). Molar mass of water = 18 g mol-1
∴ Number of moles present in 1000 g of water = \(\frac{1000}{18}=55.56 \mathrm{~mol}\)
Therefore, the mole fraction of the solute in the solution is x2 = \(\frac{1}{1+55.56}=0.0177\)
It is given that.
Vapour pressure of water, P01 = 12.3 kPa
Applying the relation.
⇒ \(\frac{p_1^0-p}{p_1^0}=x_2\)
⇒ \(\frac{12.3-\mathrm{p}_1}{12.3}=0.0177\)
⇒ 12.3 -P1 = 0.2177
⇒ p1 = 12.0823 = 12.08 kPa (approximately)
Hence, the vapour pressure of the solution is 12.08 kPa.
Question 30. Calculate the mass of a non-volatile solute (molar mass 40 g mol-1) which should be dissolved in in 114 g octane to reduce its vapour pressure to 80%.
Answer:
Let the vapour pressure of pure octane be p01.
Then, the vapour pressure of the octane after dissolving the non-volatile solute is
⇒ \(\frac{80}{100} p_1^{0}=0.8 p_1^0\)
Molar mass of solute, M2 = 40 g mol-1
Mass of octane, w1 = 114 g
Molar mass of octane, (C8H18)M1 = 8 x 12 + 18 x 1 = 114 g mol-1
Applying the relation,
⇒ \(\frac{p_1^0-p_1}{p_1^0}=\frac{w_2 \times M_1}{M_2 \times w_1}\)
⇒ \(\frac{p_1^0-0.8 p_1}{p_1^0}=\frac{w_2 \times 114}{40 \times 114}\)
⇒ \(\frac{0.2 \mathrm{p}_1^0}{\mathrm{p}_1^0}=\frac{w_2}{40}\)
⇒ \(0.2=\frac{W_2}{40}\)
W2 = 8g
Hence, the required mass of the solute is 8g.
Question 31. A solution containing 30 g of non-volatile solute exactly in 90 g of water has a vapour pressure of 2.8 kPa at 298 K. Further, 18 g of water is then added to the solution and the new vapour the pressure becomes 2.9 kPa at 298 K. Calculate:
- The molar mass of the solute
- Vapour pressure of water at 298 K.
Answer:
1. Let, the molar mass of the solute be M g mol-1
Now, the no, of moles of solvent (water), n1 = \(\frac{90 \mathrm{~g}}{18 \mathrm{~g} \mathrm{~mol}^{-1}}=5 \mathrm{~mol}\)
And, the no. of moles of solute, n2 \(\frac{30 \mathrm{~g}}{\mathrm{M} \mathrm{mol}^{-1}}=\frac{30}{\mathrm{M}} \mathrm{mol}\)
p1 = 2.8 kPa
Applying the relation: \(\frac{p_1^0-p}{p_1^0}=\frac{n_2}{n_1+n_2}\)
⇒ \(\frac{p_1^0-2.8}{p_1^0}=\frac{\frac{30}{M}}{5+\frac{30}{M}}\)
⇒ \(\frac{\mathrm{p}_1^0}{2.8}=\frac{5 \mathrm{M}+30}{5 \mathrm{M}}\)
After the addition of 18 g of water:
⇒ \(n_1=\frac{90+18 g}{18}=6 \mathrm{~mol}\)
P1 = 2.9 kPa
Again, applying the relation: \(\frac{p_1^0-p_1}{p_1^0}=\frac{n_2}{n_1+n_2}\)
⇒ \(\frac{p_1^0-2.9}{p_1^0}=\frac{\frac{30}{M}}{6+\frac{30}{M}}\)
⇒ \(\frac{p_1^0}{2.9}=\frac{6 M+30}{6 M}\)
Dividing equation (1) by (2), we have:
⇒ \(\frac{2.9}{2.8}=\frac{\frac{5 M+30}{5 M}}{\frac{6 M+30}{6 M}}\)
⇒ \(\frac{2.9}{2.8} \times \frac{6 \mathrm{M}+30}{6}=\frac{5 \mathrm{M}+30}{5}\)
⇒ 2.9 x 5 x (6M + 30) = 2.8 x 6 x (5M + 30)
⇒ 87 M + 435 = 84M + 504
⇒ 3M = 69
⇒ M = 23u
Therefore, the molar mass of the solute is 23 g mol-1.
2. Putting the value of ‘M’ in equation (1), we have:
⇒ \(\frac{\mathrm{p}_1^0}{2.8}=\frac{5 \times 23+30}{5 \times 23}\)
⇒ \(\frac{p_1^0}{2.8}=\frac{145}{115}\)
⇒ \(p_1^0=3.53\)
Hence, the vapour pressure of water at 298 K is 3.53 kPa.
Question 32. A 5% solution (by mass) of cane sugar in water has a freezing point of 271 K. Calculate the freezing point of 5% glucose in water if the freezing point of pure water is 273.15 K.
Answer: Here, ΔTf = (273.1 5- 271)K = 2.15 K
Molar mass of sugar (C12H22O11) =12 × 12 + 22 × 1 + 11 × 16 = 342 g mol-1
5% solution (by mass) of cane sugar in water means 5 g of cane sugar is present in (100- 5)g = 95g of water.
Now, number of moles of cane sugar = \(\frac{5}{342} \mathrm{~mol}=0.0146 \mathrm{~mol}\)
Therefore, molality of the solution, m = \(\frac{0.0146 \mathrm{~mol}}{0.095 \mathrm{~kg}}\) = 0.1537 mol kg-1
Applying the relation.
ΔTf = Kf × m
⇒ \(\mathrm{K}_{\mathrm{f}}=\frac{\Delta \mathrm{T}_{\mathrm{f}}}{\mathrm{m}}=\frac{2.15 \mathrm{~mol}}{0.1537 \mathrm{~mol} \mathrm{~kg}^{-1}}\) = 13.99K kg mol-1
Molar mass of glucose (C6H12O6) = 6 × 12+12 × 12 × 1 + 6 × 16= 180 g mol-1.
5% glucose in water means 5g of glucose is present in (100- 5) = 95 g of water.
∴ Number of moles of glucose = \(\frac{5}{180} \mathrm{~mol}=0.0278 \mathrm{~mol}\)
Therefore, molality of the solution, \(\mathrm{m}=\frac{0.0278 \mathrm{~mol}}{0.095 \mathrm{~kg}}=0.2926 \mathrm{mpl} \mathrm{kg}^{-1}\)
Applying the relation,
ΔTf = Kf × m
⇒ 13.99 K kg mol-1 × 0.2926 mol kg-1
⇒ 4.09 K (approximately)
Hence, the freezing point of the 5% glucose solution is (273. 1 5- 4.09) K = 269.06 K
Question 33. Two elements A and B form compounds having the formula AB2 and AB4. When dissolved in 20 g of benzene (C6H6). 1 g of AB2 lowers the freezing point by 2.3 K whereas 1.0 g of AB4 lowers it by 1,3K. The molar depression constant for benzene is 5.1 K kg mol-1. Calculate the atomic masses of A and B.
Answer:
We know that M2 = {latex]\frac{1000 \times w_2 \times k_f}{\Delta T_f \times w_1}[/latex]
Then, MAB2 = \(\frac{1000 \times 1 \times 5.1}{2.3 \times 20}=110.86 \mathrm{~g} \mathrm{~mol}^{-1}\)
MAB4 = \(\frac{1000 \times 1 \times 5.1}{1.3 \times 20}=196.15 \mathrm{~g} \mathrm{~mol}^{-1}\)
Now, we have the molar masses of AB2 and AB4 as 110.87 g mol-1 and 196.15 g mol-1 respectively.
Let the atomic masses of A and B be x and y respectively.
Now, we can write:
x + 2y = 110.87 ____ 1
x + 4y= 196.15 _____ 2
Subtracting equation 1 from 2, we have
2y = 85.28
y = 42.64
Putting the value of ‘y’ in equation (1), we have
x + 2 × 42.64= 110.87
x = 25.59
Hence, the atomic masses of A and B are 25.59 u and 42.64 u respectively.
Question 34. At 300 K. 36g of glucose present in a litre of its solution has an osmotic pressure of 4.98 bar. If the osmotic pressure of the solution is 1.52 bars at the same temperature, what would be its concentration?
Answer:
Here, T = 300 K:
p = 1.52 bar:
R = 0.083 bar L K-1 mol-1
Applying the relation.
π = CRT
⇒ \(\mathrm{C}=\frac{\pi}{\mathrm{RT}}=\frac{1.52}{0.083 \mathrm{bar}, \mathrm{L} \cdot \mathrm{K}^{-1} \mathrm{~mol}^{-1} \times 300 \mathrm{~K}}=0.061 \mathrm{~mol}\)
Since the volume of the solution is 1 L, the concentration of the solution would be 0.061 M.
Question 35. Suggest the most important type of intermolecular attractive interaction in the following pairs.
- N-hexane and n-oetane
- I2 and CCI4
- NaClO4, and water (H2O)
- Methanol and acetone
- Acetonitrile (CH3CN) and acetone (C3H6O).
Answer:
- Van der Wall’s forces of attraction.
- Van der Wall’s forces of attraction.
- Ion-dipole interaction.
- Dipole – dipole interaction.
- Dipole – dipole interaction
Question 36. Based on solute-solvent interactions, arrange the following in order of increasing solubility in n-octane and explain. Cyclohexane, KCl, CH3OH, CH3CN.
Answer:
N-octane is a non-polar solvent. Therefore, the solubility of a non-polar solute is more than that of
a polar solute in the n-octane.
The order of increasing polarity is: Cyclohexane < CH3CN < CH3OH < KCI
Therefore, the order of increasing solubility is: KCI < CH OH < CH3CN < Cyclohexane.
Question 37. Amongst the following compounds, identify which are insoluble, partially soluble and highly soluble in water.
- Phenol
- Toluene
- Formic acid
- Ethylene glycol
- Chloroform
- Pentanol,
Answer:
- Phenol (C6H5OH) has the polar group -OH and non-polar group -C6H5. Thus, phenol is partially soluble in water.
- Toluene (C6H5CH3.) has no polar group. Thus, toluene is insoluble in water
- Formic acid (HCOOH) lias the polar group -OH and can form an H-bond with water. Thus, formic acid is highly soluble in water.
- Ethylene glycol
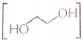 has a polar -OH group and can form an H-bond. Thus, it is highly soluble in water
has a polar -OH group and can form an H-bond. Thus, it is highly soluble in water - Chloroform is insoluble in water.
- Pentanol (C5H11OH) has a polar -OH group, but it also contains a very bulky non-polar -C5H11 group. Thus, pentanol is partially soluble in water.
Question 38. If the density of some lake water is 1.25 g mL-1 and contains 92 g of Na+ ions per kg of water, calculate the molality of Na+ ions in the lake.
Answer:
Number of moles present in 92 g of Na+ ions
⇒ \(\frac{92 \mathrm{~g}}{23 \mathrm{~g} \mathrm{~mol}^{-1}}=4 \mathrm{~mol}\)
Therefore, the molality of Na+ ions in the lake
⇒ \(\frac{4 \mathrm{~mol}}{1 \mathrm{~kg}}=4 \mathrm{~m}\)
Question 39. If the solubility product of CuS is 6 × 106, calculate the maximum molarity of CuS in aqueous solution.
Answer:
Solubility product of CuS, Ksp = 6 × 10-16
Let s be the solubility of CuS is mol L-1
⇒ \(\mathrm{CuS} \longleftrightarrow \mathrm{Cu}_{\mathrm{S}}^{2+}+\mathrm{S}_{\mathrm{S}}^{2-}\)
Now, Ksp = [Cu2+][S2+]
= S × S = S2
Then, we have, K = S2 = 6 x 10-16
⇒ \(\mathrm{s}=\sqrt{6 \times 10^{-16}}=2.45 \times 10^{-8} \mathrm{~mol} \mathrm{~L}^{-1}\)
Hence, the maximum molarity of CuS in an aqueous solution is 2.45 x 10-8 mol L-1
Question 40. Calculate the mass percentage of aspirin (C9H8O4) in acetonitrile (CH3CN) when 6.5 g of C9H8O4 is dissolved in 450 g of CH3CN.
Answer:
6.5 g of aspirin (C9H8O4) is dissolved in 450 g of acetonitrile (CH3CN)
Then, total mass of the solution = (6.5 + 450) g = 456.5 g
Therefore, mass percentage of C9H8O4 = \(\frac{6.5}{456.5} \times 100 \%=1.424 \%\)
Question 41. Nalorphcne (C19H21NO3), similar to morphine, is used to combat withdrawal symptoms in narcotic users. The dose of nalorphine generally given is 1.5 mg. Calculate the mass of the 1.5 x 10 3 m aqueous solution required for the above dose.
Answer:
The molar mass of nalorphine (C19H21NO3) is given as:
19 × 12 + 21 × 1 + 1 × 14 + 3 × 16 = 311 g mol-1
In 1.5 × 10-3 m aqueous solutions of nalorphine,
1 kg (1000 g) of water contains 1.5 × 10-3 mol = 1.5 × 10-3 × 311 g = 0.4665 g nalorphine
Therefore, total mass of the solution = (1000 + 0.4665) g = 1000.4665 g
This implies that the mass of the solution containing 0.4665 g of nalorphine is 1000.4665 g.
Therefore, the mass of the solution containing 1.5 mg of nalorphine is:
⇒ \(\frac{1000.4665 \times 1.5 \times 10^{-3}}{0.4665} \mathrm{~g}=3.22 \mathrm{~g}\)
Hence, the mass of aqueous solution required is 3.22 g
Note: There is slight variation in this answer and the one given in the Ncert textbook
Question 42. Calculate the amount of benzoic acid (C6H12COOH) required for preparing 250 mh. of 0.15 M solution in methanol.
Answer:
0.15 M solution of benzoic acid in methanol means, 1000 mL of solution contains 0.15 mol of benzoic acid.
Therefore. 250 mL of solution contains = \(\frac{0.15 \times 250}{1000} \mathrm{~mol} \text { of benzoic acid }\)
= 0.0375 mol of benzoic acid
Molar mass of benzoic acid (C6H5COOH) = 7 × 12 + 6 x 1 + 2 × 16 = 122 g mol-1
Hence, required benzoic acid = 0.0375 mot × 122 g mol-1 = 4.575 g
Question 43. The depression in the freezing point of water observed for the same amount of acetic acid, trichloroacetic acid and trifluoroacetic acid increases in the order given above. Explain briefly.
Answer:
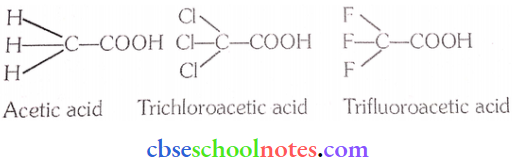
- Among H, Cl, and F, H is the least electronegative while F is the most electronegative. Then, F can withdraw electrons towards itself more than Cl and H.
- Thus, trifluoroacetic acid can easily lose H+ ions i.e. trifluoroacetic acid ionizes to the largest extent. Now. the more ions produced, the greater the depression of the freezing point. Hence, the depression in the freezing point increases in the order.
Acetic acid < trichloroacetic acid < trifluoroacetic acid
Question 44. Calculate the depression in the freezing point of water when 10 g, CH3 CH2CHClCOOH is added to 250 g of water, K a = 1.4 x 10-3, Kf = 1.86 K kg mol-1.
Answer:
Molar mass of CH3CH2CHCICOOH = 15 + 14+ 13 + 35.5 + 12 + 16 + 16 + 1 = 122.5 g mol-1
No. of moles present in 10 g CM CH CHCICOOH = \(\frac{10 \mathrm{~g}}{122.5 \mathrm{~g} \mathrm{~mol}^{-1}}=0.0816 \mathrm{~mol}\)
It is given that 10 g CH3CH2CHCICOOH is added to 250 g of water.
The molality of the solution. = \(\frac{0.0186}{250} \times 1000=0.3265 \mathrm{~mol} \mathrm{~kg}^{-1}\)
Let a be the degree of dissociation of CH3CH2CHCICOOH
CH3CH2CHCICOOH undergoes dissociation according to the following equation:
CH3CH2CHCICOOH ↔ CH3CH2CHClCOO– + H+
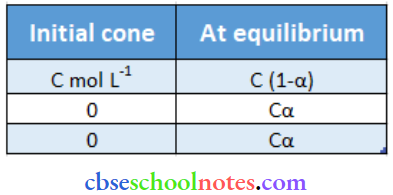
⇒ \(K_a=\frac{C \alpha \cdot C \alpha}{C(1-\alpha)}=\frac{C \alpha^2}{(1-\alpha)}\)
Since a is very small concerning 1, 1 – α ≈ 1
Now, ⇒ K = Cα2
⇒ \(\alpha=\sqrt{\frac{\mathrm{K}_{\mathrm{a}}}{\mathrm{C}}}=\sqrt{\frac{1.4 \times 10^{-3}}{0.3264}}=0.0655 \quad\left(\mathrm{~K}_{\mathrm{a}}=1.4 \times 10^{-3}\right)\)
Again, CH3CH2CHCICOOH ↔ CH3CH2CHCICOO– + H+
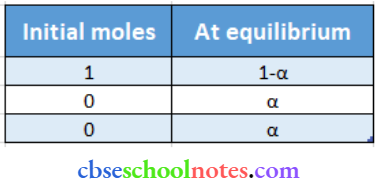
Total mass of equilibrium = I – α + α + α = 1 + α
⇒ \(\mathrm{i}=\frac{1+\alpha}{1}=1+\alpha=1+0.0655=1.0655\)
Hence, the depression in the freezing point of water is given as:
ΔTf = i.Kfm = 1.0655 × 1.86 kg mol-1 × 0.364 mol kg-1=0.65 K
Question 45. 19.5 g of CH2FCOOH is dissolved in 500 g of water. The depression in the freezing point of water observed is 1.0°C. Calculate the van’t a Hoff factor and dissociation constant of fluoro acetic acid.
Answer:
It is given that:
w1 = 500 g,
w2 = 19.5 g
Kf = 1.86 K kg mol-1
ΔTf = 1 K
We know that: \(\mathrm{M}_2=\frac{\mathrm{K}_f \times \mathrm{w}_2 \times 1000}{\Delta \mathrm{T}_{\mathrm{f}} \times \mathrm{w}_1}\)
⇒ \(=\frac{1.86 \mathrm{~K} \mathrm{~kg} \mathrm{~mol}^{-1} \times 19.5 \mathrm{~g} \times 1000 \mathrm{~g} \mathrm{~kg}^{-1}}{500 \mathrm{~g} \times 1 \mathrm{~K}}\) = 72.54mol-1
Therefore, observed molar mass of CH2FCOOH, (M2)obs = 72.54 mol
The calculated molar mass of CH2FCOOH is:
(M2)cal = 14 +19 + 12 + 16+ 16+ 1 = 78 g mol-1
Therefore, van’t Hoff factor,
⇒ i = \(\frac{\left(\mathrm{M}_2\right)_{\text {cat }}}{\left(\mathrm{M}_2\right)_{\text {obo }}}=\frac{78 \mathrm{~g} \mathrm{~mol}^{-1}}{72.54 \mathrm{~g} \mathrm{~mol}^{-1}}=1.0753\)
Let a be the degree of dissociation of CH2FCOOH
⇒ \(\mathrm{CH}_2 \mathrm{FCOOH} \quad \longleftrightarrow \quad \mathrm{CH}_2 \mathrm{FCOO}^{-}+\mathrm{H}^{+}\)
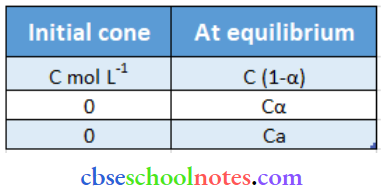
Total = C (1 + α)
⇒ \(\mathrm{i}=\frac{\mathrm{C}(1+\alpha)}{\mathrm{C}}\)
⇒ i = 1 + α
⇒ α = i – 1 = 1.0753 – 1 = 0.0753
Now. the value of Ka is given as:
⇒ \(\mathrm{K}_{\mathrm{a}}=\frac{\left[\mathrm{CH}_2 \mathrm{FCOO}^{-}\right]\left[\mathrm{H}^{-}\right]}{\left[\mathrm{CH}_2 \mathrm{FCOOH}\right]}=\frac{\mathrm{C} \alpha \cdot \mathrm{C} \alpha}{\mathrm{C}(1-\alpha)}=\frac{\mathrm{C}^2}{1-\alpha}\)
Take the volume of the solution as 500 mL.
We have a concentration: of 19.5 M
weight = 19.5 g
Concentration = \(\frac{\frac{19.5}{78}}{500} \times 1000=0.5 \mathrm{M}\)
Therefore, Ka = \(\frac{\mathrm{C} \alpha^2}{1-\alpha}\)
⇒ \(\frac{0.5 \times(0.0753)^2}{1-0.0753}=\frac{0.5 \times 0.00567}{0.9247}=0.00307 \text { (approximately) }\)
= 3.07 x 10-3
Question 46. The vapour pressure of water at 293 K is 17.535 mm Hg. Calculate the vapour pressure of water at 293 K when 25 g of glucose is dissolved in 450 g of water.
Answer:
Vapour pressure of water, p01 = 17.535 mm of Hg
Mass of glucose, w2 = 25 g
Mass of water, w1 = 450 g
We know that.
Molar mass of glucose (C6H12O6).M2 = 6 x 12 + 12 x 1 + 6 x 16 = 180 g mol-1
The molar mass of water. M1 = 18 g mol-1
Then, number of moles of glucose, n2 = \(\frac{25}{180 \mathrm{~g} \mathrm{~mol}^{-1}}=0.139 \mathrm{~mol}\)
And. number of moles of water, n1 = \(\frac{450 \mathrm{~g}}{18 \mathrm{~g} \mathrm{~mol}^{-1}}=25 \mathrm{~mol}\)
We know that,
⇒ \(P_1=P^0 \times\left[\frac{n_1}{n_1+n_2}\right]\)
⇒ \(P_1=17.535\left[\frac{25}{25+0.139}\right]=\frac{17.535 \times 25}{25.139}\)
= 17.535 -p1 = 0.097
= p1 = 17.44 mm of Hg
Hence, the vapour pressure of water is 17.44 mm of Hg.
Question 47. Henry’s law constant for the molality of methane in benzene at 298 K is 4.27 x 10-3 mm Hg. Calculate the solubility of methane in benzene at 298 Kelvine 760 mm Hg.
Answer:
Here, p = 760 mm Hg;
kH = 4.27 x 105 mm Hg
According to Henry’s law,
p = KHX \(\Rightarrow \quad x=\frac{p}{k_{H}}=\frac{760 \mathrm{~mm} \mathrm{Hg}}{4.27 \times 10^5 \mathrm{~mm} \mathrm{Hg}}\)
= 177.99 x 10-5 = 178 x 10-5 (approximately)
Hence, the mole fraction of methane in benzene is 178 x 10-5
Question 48. 100g of liquid A (molar mass 140 g mol-1) was dissolved in 1000 g of liquid B (molar mass 180 g mol-1). The vapour pressure of pure liquid B was found to be 500 torr. Calculate the vapour pressure of pure liquid A and its vapour pressure in the solution if the total vapour pressure of the solution is 475 Torr.
Answer:
Number of moles of liquids A, nA= \(\frac{100}{140} \mathrm{~mol}=0.714 \mathrm{~mol}\)
Number of moles of liquids B, nB = \(\frac{1000}{180} \mathrm{~mol}=5.556 \mathrm{~mol}\)
Then, the mole fraction of A
⇒ \(x_A=\frac{n_A}{n_A+n_B}=\frac{0.714}{0.714+5.556}=0.114\)
And, mole fraction of B, xB = 1 – 0.1 14 = 0.886
Vapour pressure of pure liquid B, p0B = 500 torr
Therefore, the vapour pressure of liquid B in the solution,
pB = P0B xB = 500 x 0.886 a 443 torr
Total vapour pressure of the solution, pTotal = 475 torr
∴ Vapour pressure of liquid A in the solution,
PA = PTotal – PB = 475 – 443 = 32 toor
Now, \(p_A=p_A^0 x_A \quad \Rightarrow \quad p_A^0=\frac{p_A}{x_A}=\frac{32}{0.114}=280.7\)
Hence, the vapour pressure of pure liquid A is 280.7 torr.
Question 49. The vapour pressure of pure acetone and chloroform at 328 K is 741.8 mm Hg and 632.8 mm Hg respectively. Assuming that they form ideal solutions over the entire range of composition, plot ptotal Pchloroform pacetone as a function of xacetone. The experimental data observed for different compositions of the mixture is:
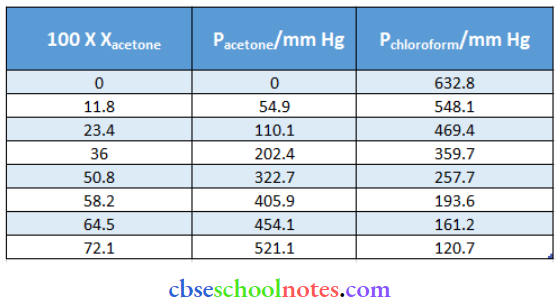
From the question, we have the following data
Answer:
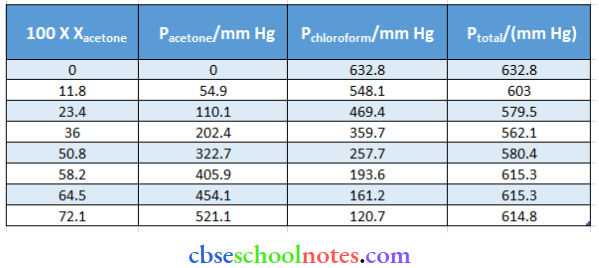
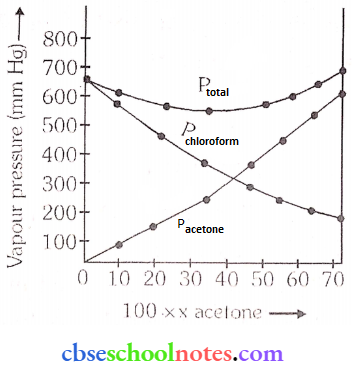
It can be observed from the graph that the plot for the plural of the solution curves downwards. Therefore, the solution shows a negative deviation from the ideal behaviour.
Question 50. Benzene and toluene form ideal solutions over the entire range of compositions. The vapour pressure of pure benzene and toluene at 300 K is 50.71 mm Hg and 32.06 mm Hg respectively. Calculate the mole fraction of benzene in the vapour phase if 80 g of benzene is mixed with 100 g of toluene.
Answer:
Molar mass of benzene (C6H6) = 6 × 12 + 6 × 1 = 78 g mol-1
Molar mass of toluene = 7 × 12 + 8 × 1 = 92 g mol-1
Now, no. of moles present in 80 g of benzene = \(\frac{80}{78} \mathrm{~mol}=1.026 \mathrm{~mol}\)
And. no. of moles present in 100 g of toluene = \(\frac{100}{92} \mathrm{~mol}=1.087 \mathrm{~mol}\)
Mole fraction of benzene, xb = \(\frac{1.026}{1.026+1.087}=0.486\)
And, mole fraction of toluene, x1 = 1 – 0.486 = 0.514
It is given that vapour pressure of pure benzene, p01 = 50.71 mm of Hg
and vapour pressure of pure toluene, p01 = 32.06 mm of Hg
Therefore, the partial vapour pressure of benzene,
p1 = x1 × p1 = 0.486 x 50.71 = 24.645 mm of Hg
And, partial vapour pressure of toluene,
p1 = x1 × p1 = 0.5 14 × 32.06 = 16.479 mm of Hg
Hence, the mole fraction of benzene in the vapour phase is given by:
⇒ \(\frac{p_b}{p_b+p_1}=\frac{24.645}{24.645+16.479}=\frac{24.645}{41.124}=0.599=0.6\)
Question 51. The air is a mixture of several gases. The major components are oxygen and nitrogen with an approximate proportion of 20% to 79% by volume at 298 K. The water is in equilibrium with air at a pressure of 10 atm. At 298 K Henry’s law constants for oxygen and nitrogen are 3,30 x 107 mm and 6.51 x 107 ram respectively. Calculate the composition of these gases in water.
Answer:
Percentage of oxygen (O2) in air = 20%
Percentage of nitrogen (N2) in air= 79%
Also, it is given that water is in equilibrium with air at a total pressure of 10 atm. that is. (10 x 760) mm Hg = 7600 mm Hg
Partial pressure of oxygen, \(\mathrm{p}_{\mathrm{O}_2}=\frac{20}{100} \times 7600 \mathrm{~mm} \mathrm{Hg}=1520 \mathrm{~mm} \mathrm{Hg}\)
Partial pressure of nitrogen, \(p_{\mathrm{N}_2}=\frac{79}{100} \times 7600 \mathrm{~mm} \mathrm{Hg}=6004 \mathrm{~mm} \text { of } \mathrm{Hg}\)
Now, according to Henry’s law : p = KHx
For oxygen: pO2 = KH.xO2
⇒ \(\mathrm{x}_{\mathrm{O}_2}=\frac{\mathrm{P}_{\mathrm{O}_5}}{\mathrm{~K}_{\mathrm{H}}}=\frac{1520 \mathrm{~mm} \text { of } \mathrm{Hg}}{3.30 \times 10^7 \mathrm{~mm} \text { of } \mathrm{Hg}}\left(\text { Given } \mathrm{K}_{\mathrm{H}}=3.30 \times 10^7 \mathrm{~mm} \text { of } \mathrm{Hg}=4.61 \times 10^5\right. \text {. }\)
For nitrogen. pN2 = KH.xN2
⇒ \(x_{\mathrm{N}_2}=\frac{\mathrm{P}_{\mathrm{N}_1}}{\mathrm{~K}_{\mathrm{H}}}=\frac{6004 \mathrm{~mm} \mathrm{Hg}}{6.51 \times 10^7 \mathrm{~mm} \mathrm{Hg}}=9.22 \times 10^{-5}\)
Hence, the mole fractions of oxygen and nitrogen in water are 4.61 × 10-5 and 9.22 × 10-5 respectively.
Question 52. Determine the amount of CaCl2 (i = 2.47) dissolved in 2.5 litres of water such that its osmotic pressure is 0.75 atm at 27°C.
Answer:
We know that, \(\pi=i \frac{n}{V} R T\)
⇒ \(\pi=i \frac{w}{M V} R T \Rightarrow w=\frac{\pi M V}{i R T}\)
⇒ π = 0.75 atm
⇒ V = 2.5 L
⇒ i = 2.47
⇒ T = (27 + 273) K = 300 K
Here,
⇒ R = 0.082 L atm K-1 mol-1
⇒ M = 1 × 40 + 2 × 35.5 = 111 g mol-1
Therefore, w = \(\frac{0.75 \times 111 \times 2.5}{2.47 \times 0.0821 \times 300}=3.42 \mathrm{~g}\)
Hence, the required amount of CaCl2 is 3.42g.
Question 53. Determine the osmotic pressure of a solution prepared by dissolving 25 mg of K2SO4 in 2 litre of water at 25°C, assuming that it is completely dissociated.
Answer: When K2SO4 is dissolved in water. K+ and SO24- ions are produced
⇒ \(\mathrm{K}_2 \mathrm{SO}_4 \longrightarrow 2 \mathrm{~K}^{+}+\mathrm{SO}_4^{2-}\)
Total number of ions produced = 3
∴ i = 3
Given, w = 25 mg = 0.025 g
V = 2 L
T = 25°C = (25 + 273) K = 298 K
Also, we know that :
R = 0.0821 L atm K-1 mol-1
M = (2 × 39) + (1 × 32) + (4 × 16) = 174 g mol-1
Applying the following relation.
⇒ \(\pi=i \frac{n}{v} R T\)
⇒ \(\mathrm{i} \frac{\mathrm{W}}{\mathrm{M}} \frac{1}{\mathrm{v}} \mathrm{RT}=3 \times \frac{0.025}{174} \times \frac{1}{2} \times 0.0821 \times 298=5.27 \times 10^{-3} \mathrm{~atm}\)
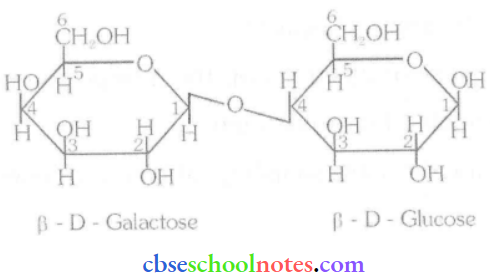
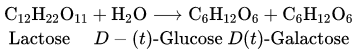
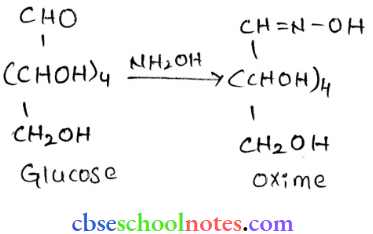
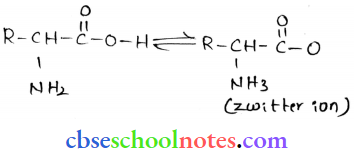
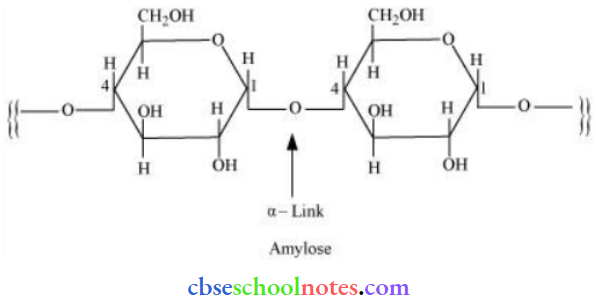

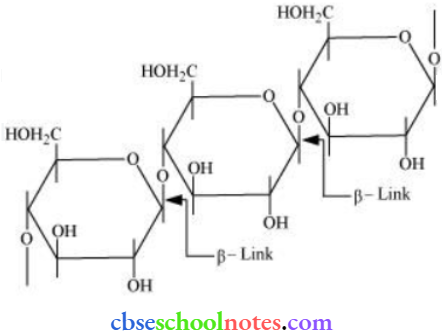
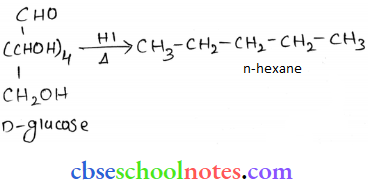
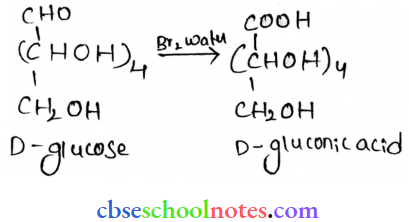
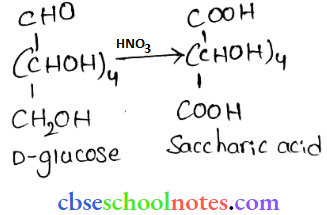
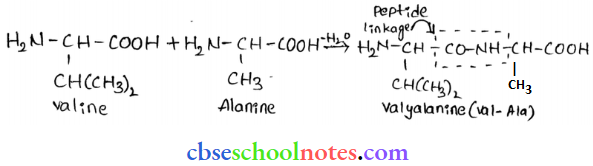
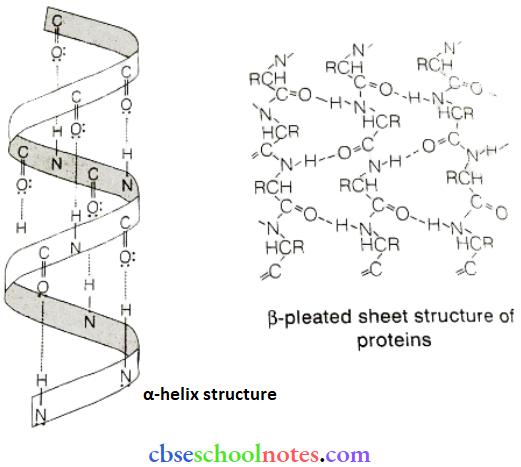
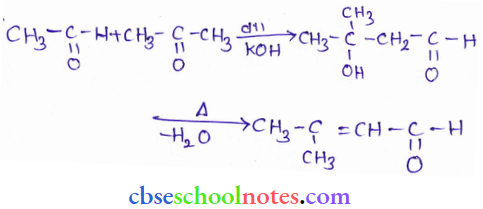
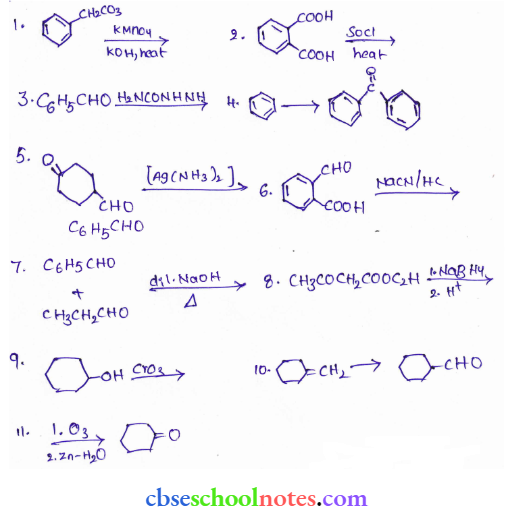
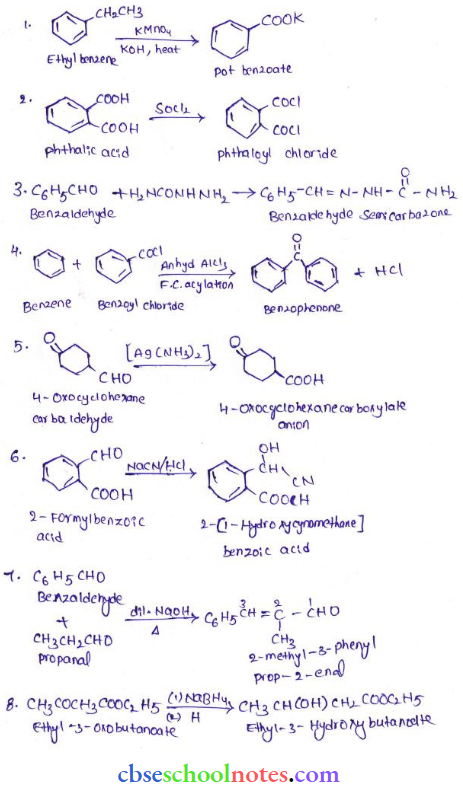
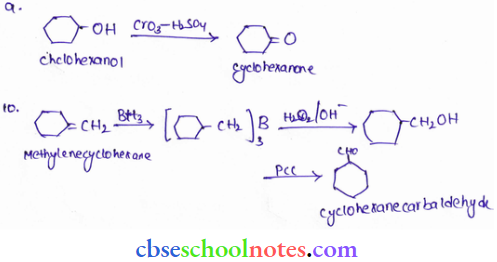
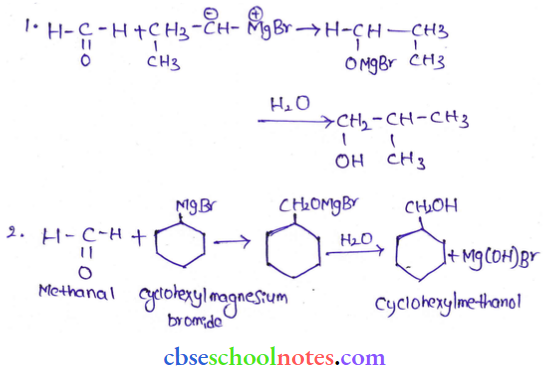
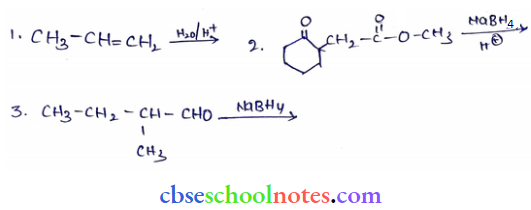
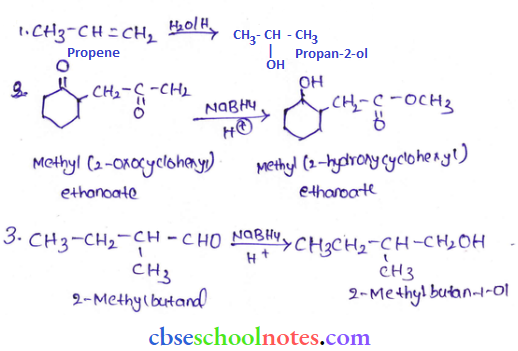
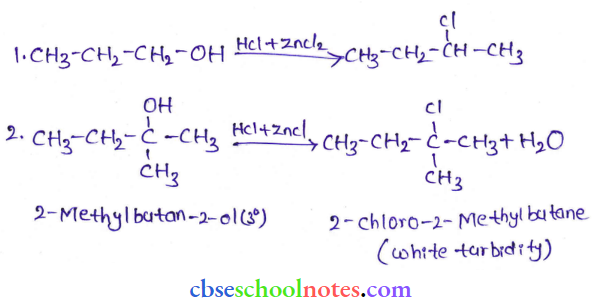
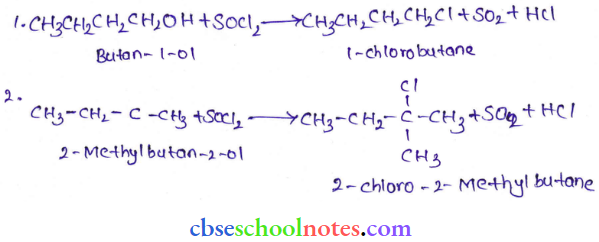
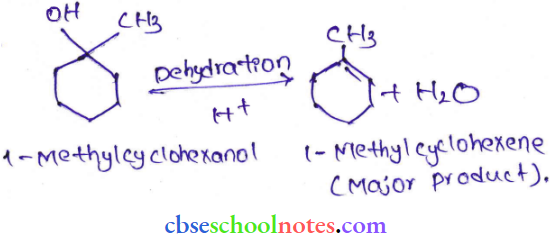
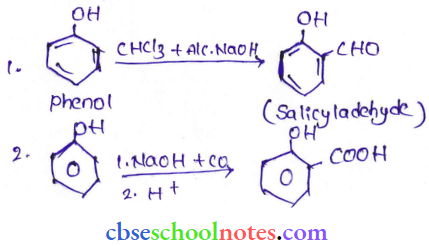
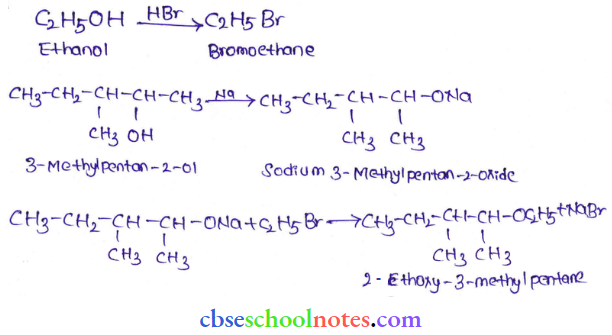
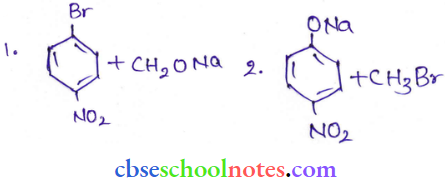
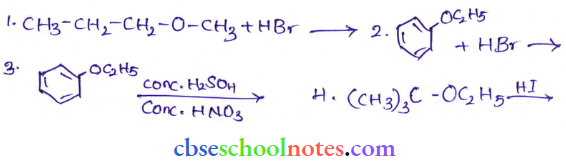
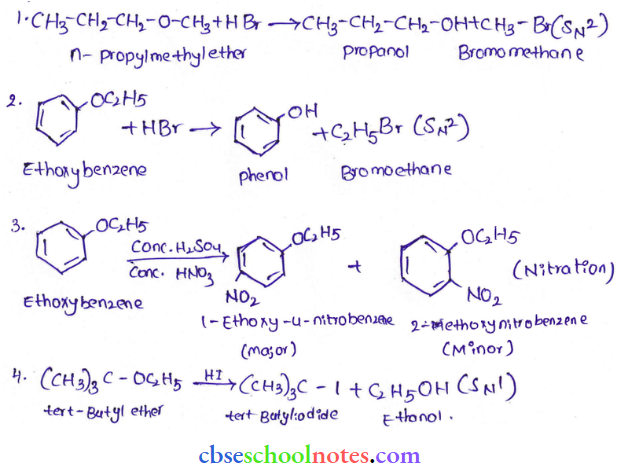
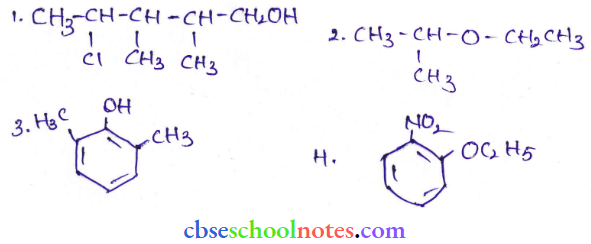
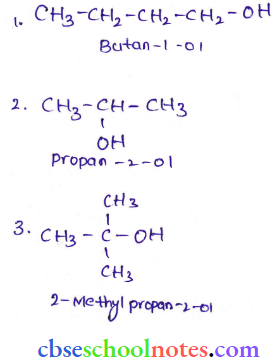
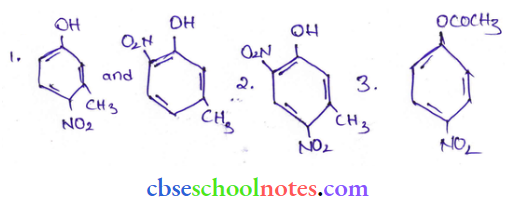
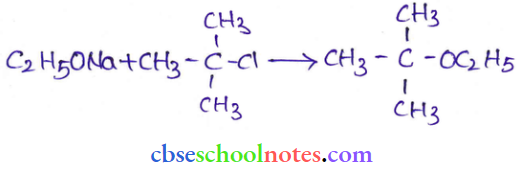
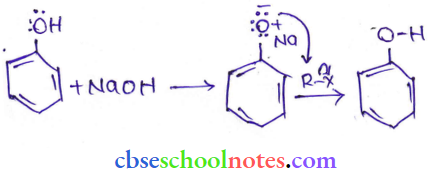
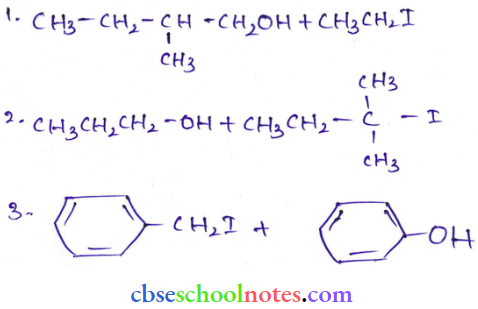
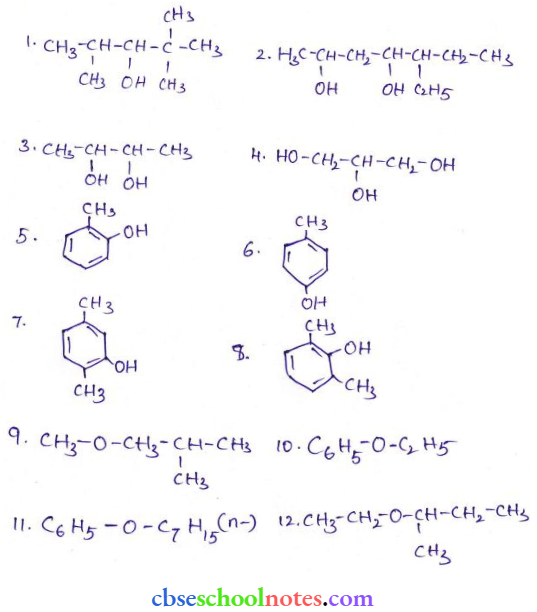
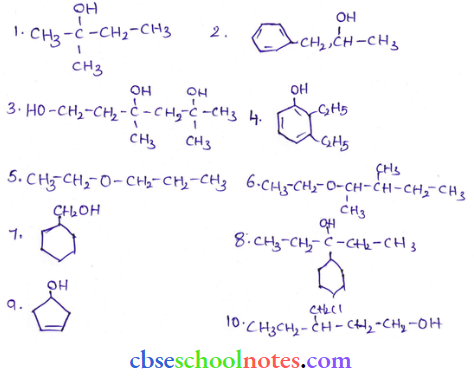
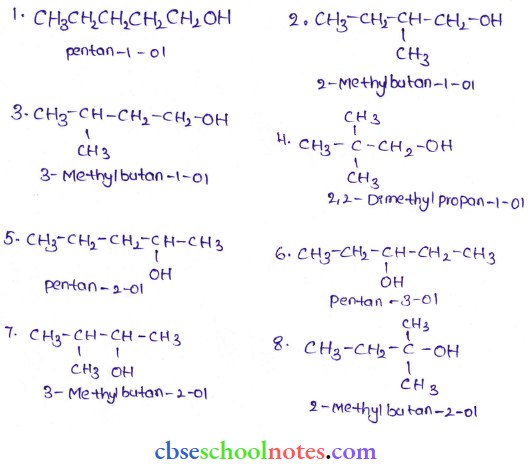
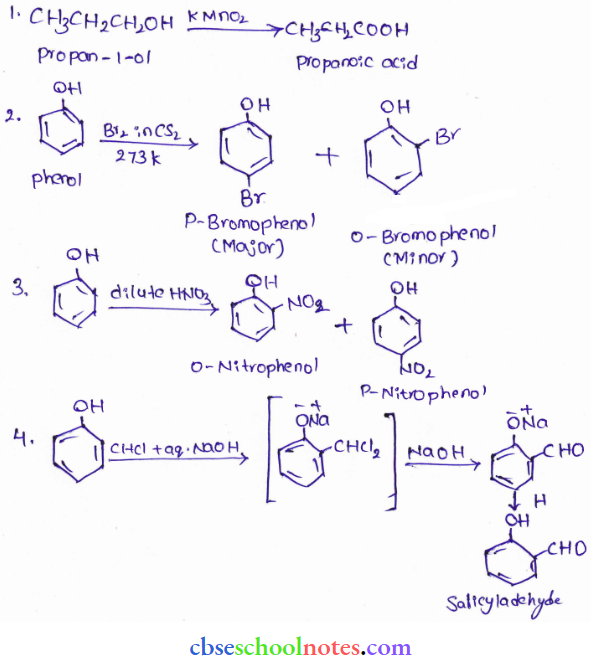
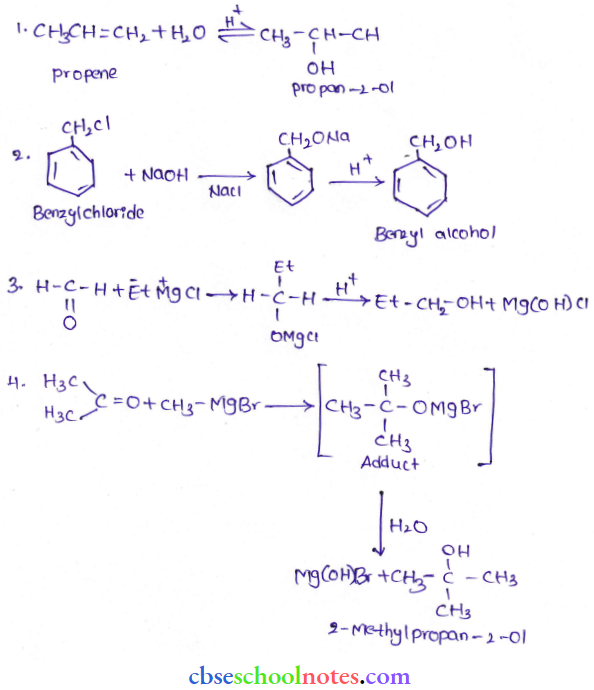
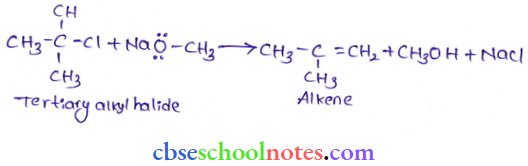
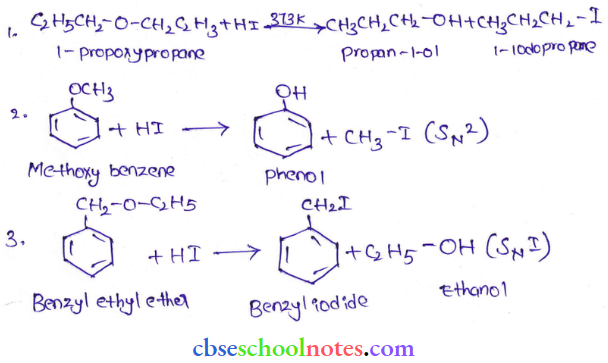
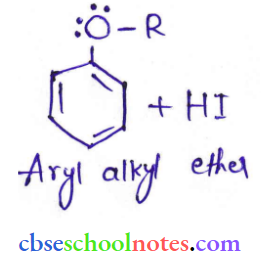
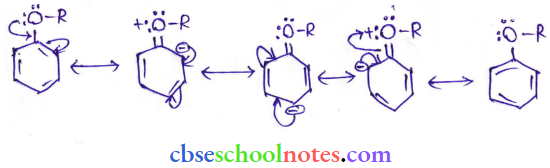
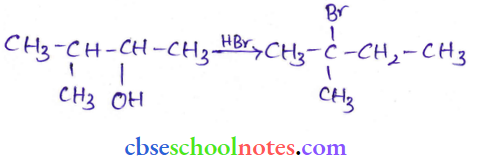
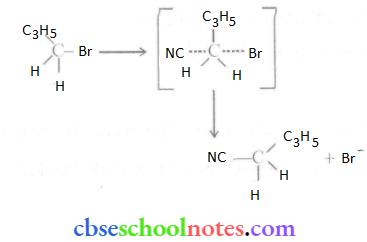
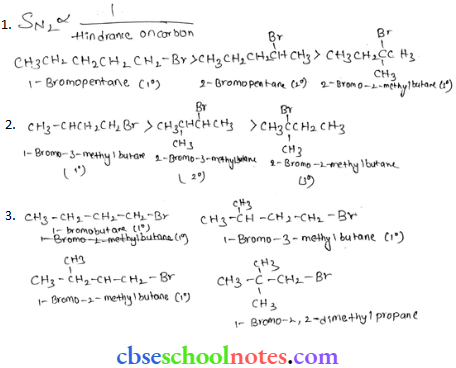
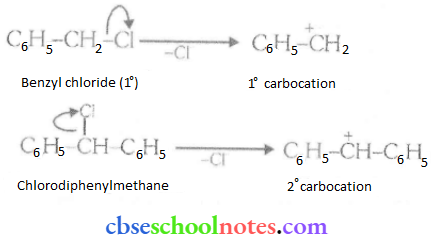
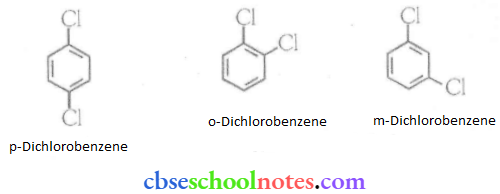
![]() group of
group of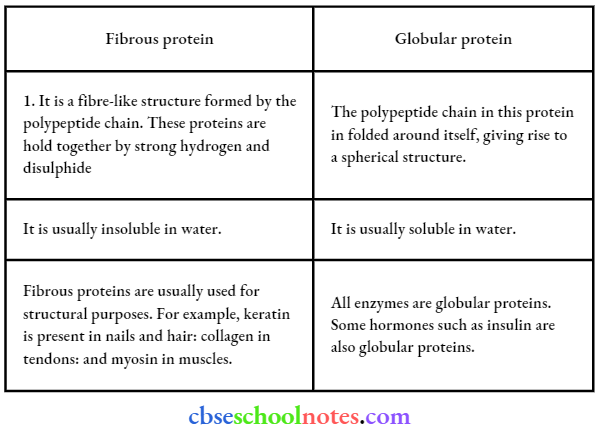
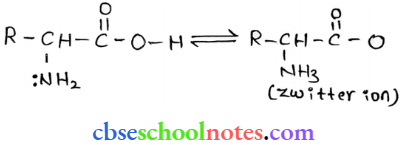
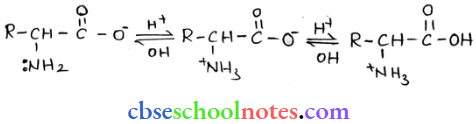
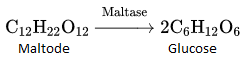


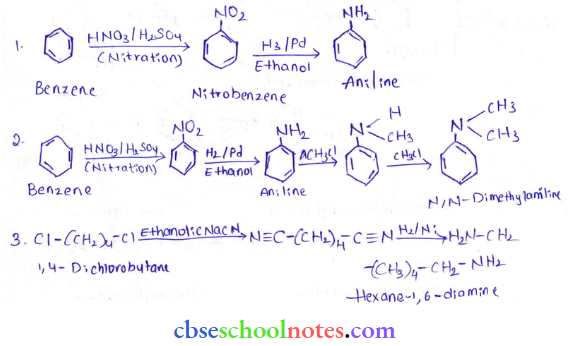
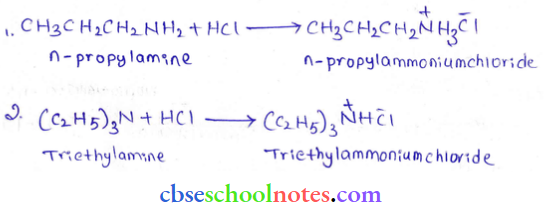
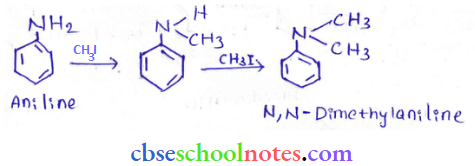
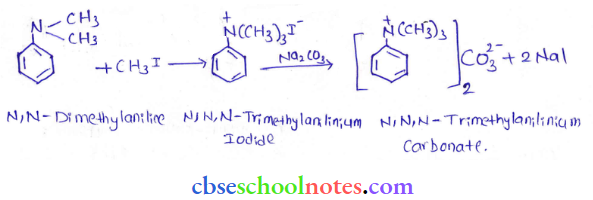
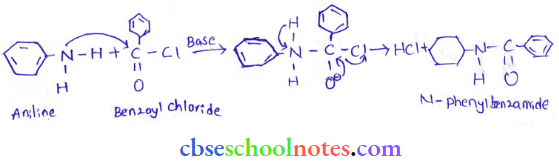
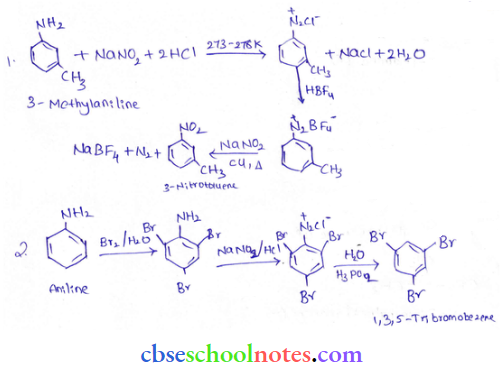
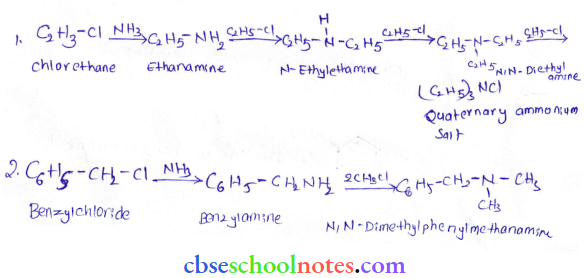
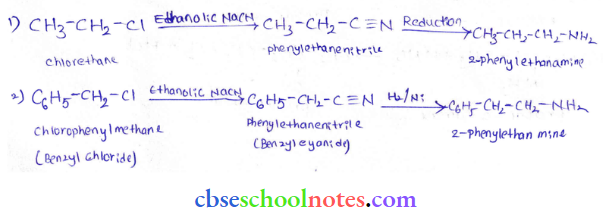
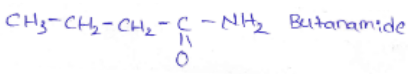
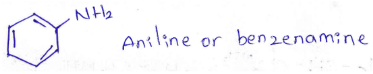
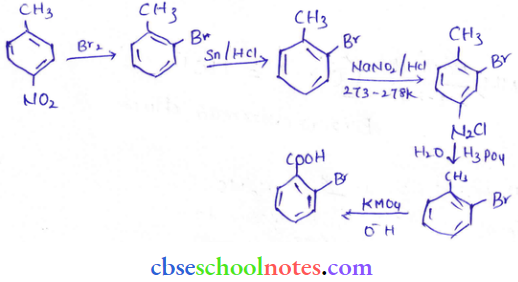
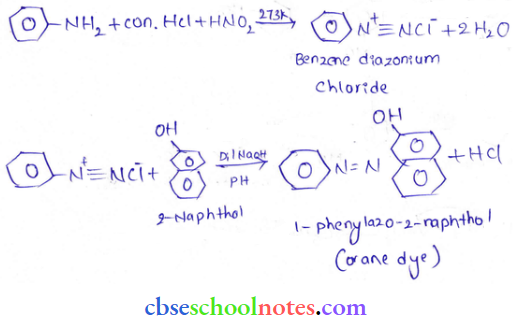
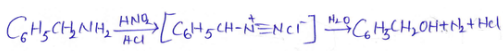
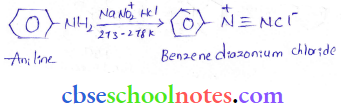
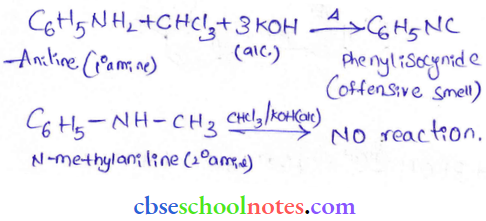
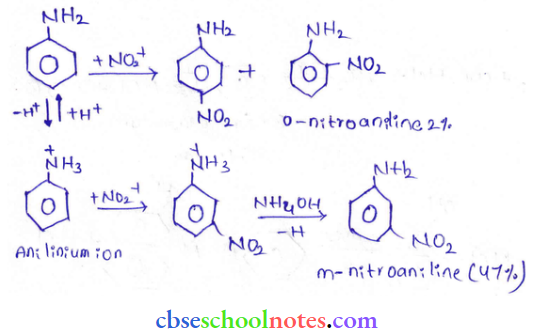


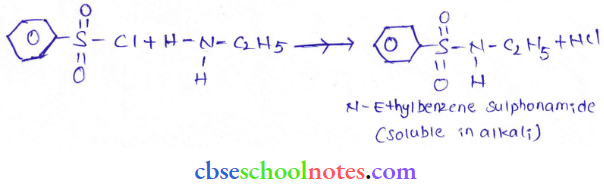
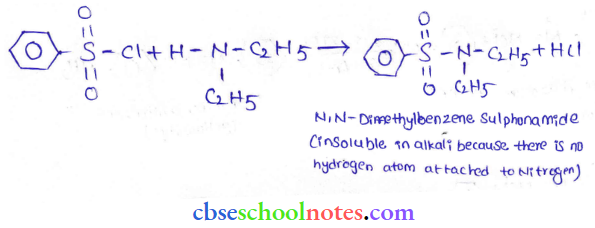

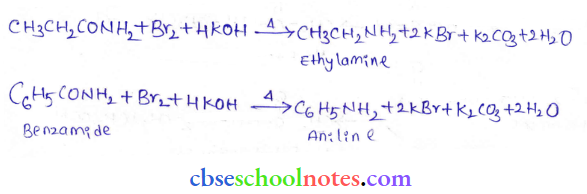
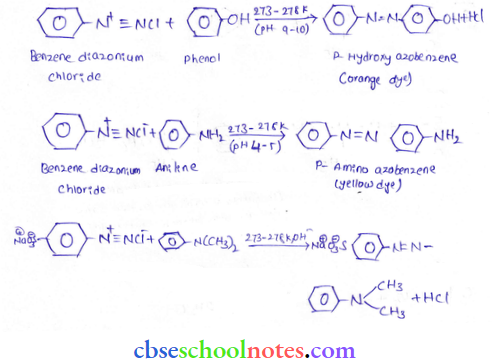
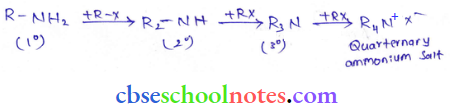
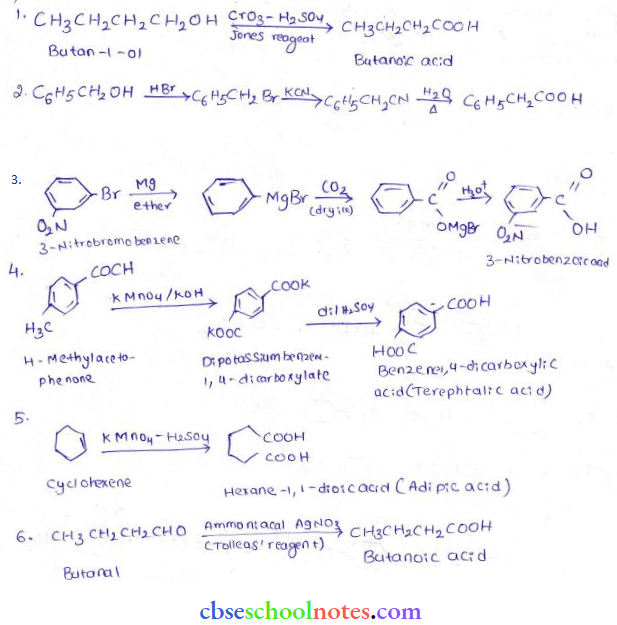
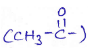 into a molecule is called acetylation. Common acetylating agents used arc acetyl chloride and acetic anhydride.
into a molecule is called acetylation. Common acetylating agents used arc acetyl chloride and acetic anhydride.
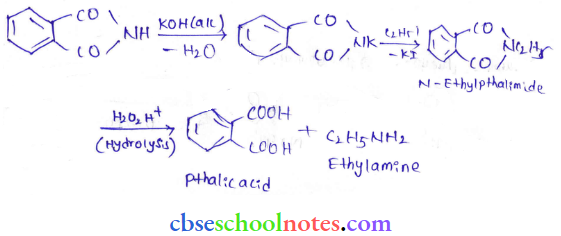
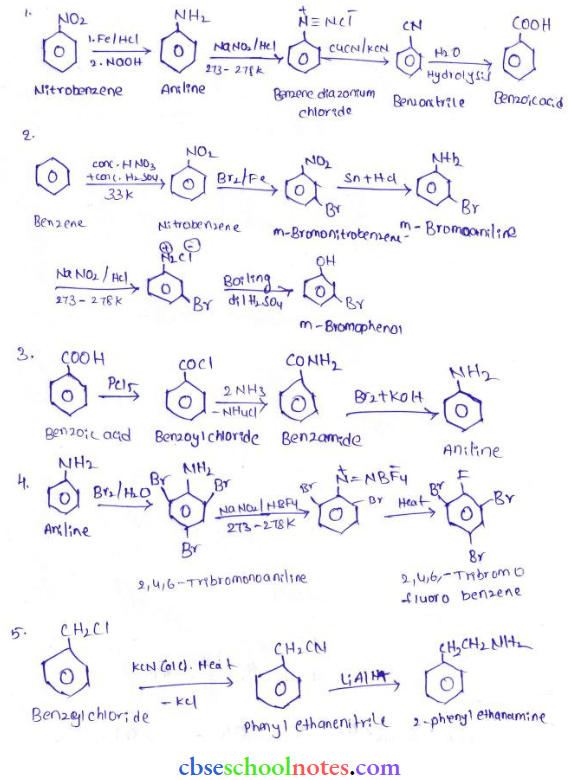
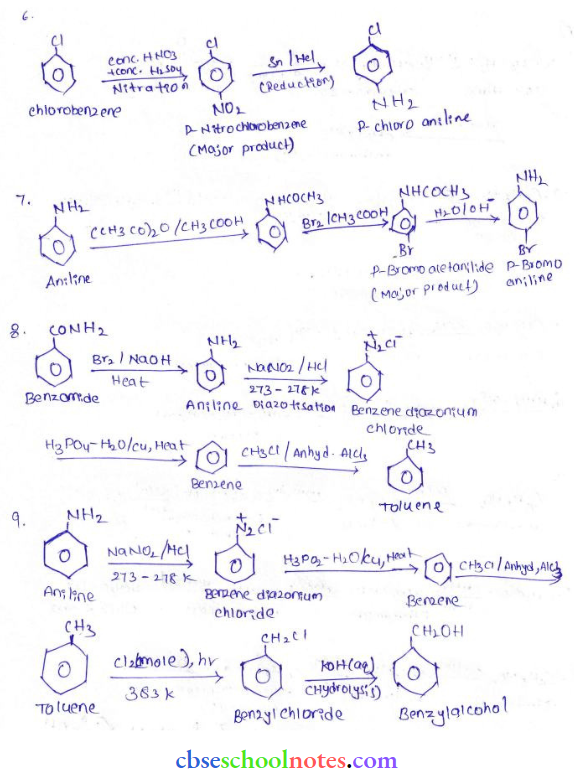




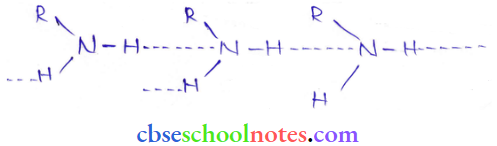
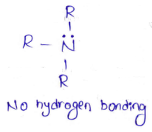
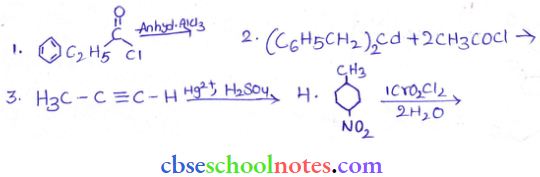
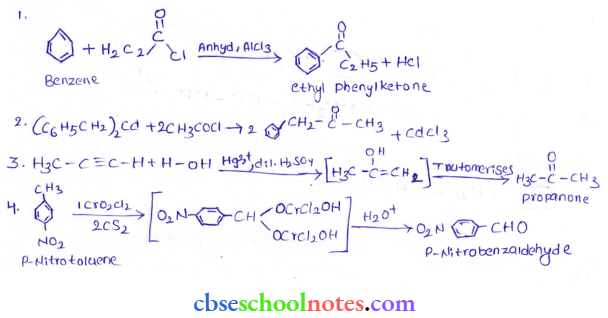
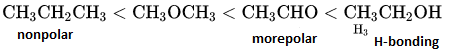
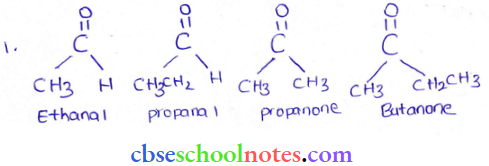
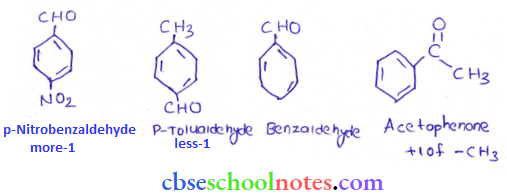
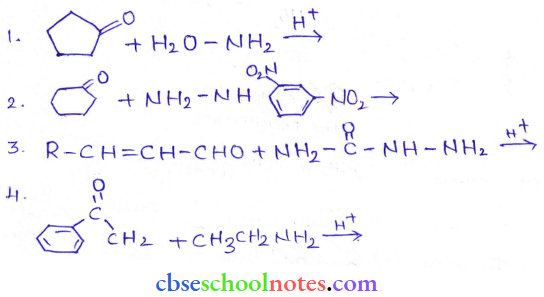
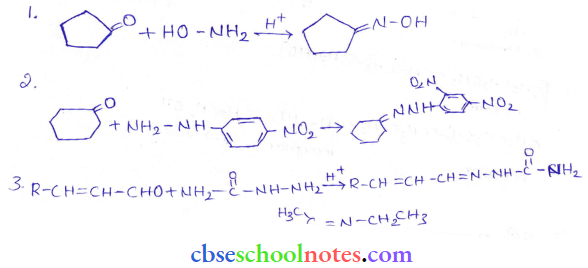
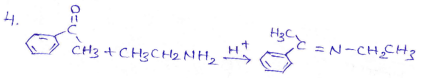
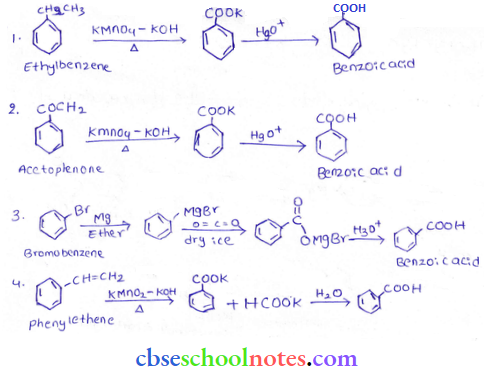

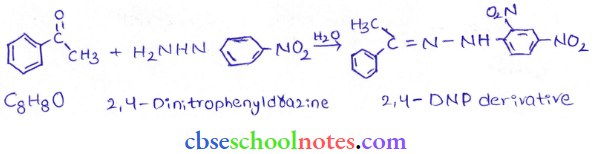

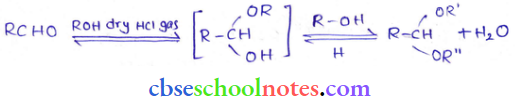
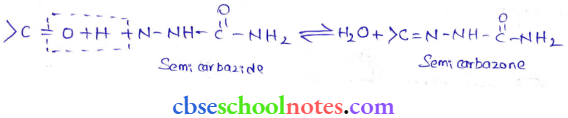
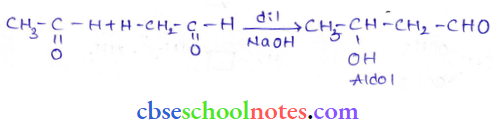
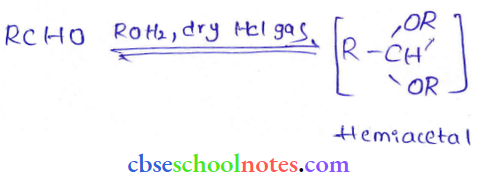
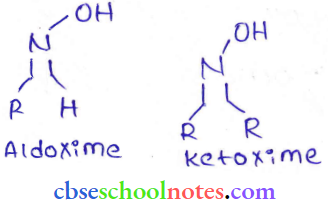
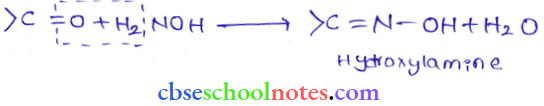
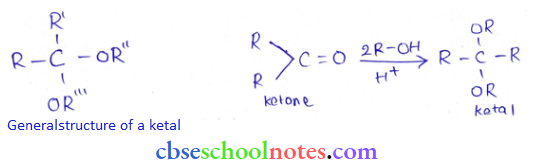



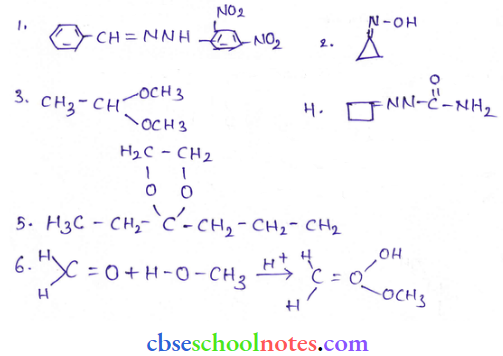
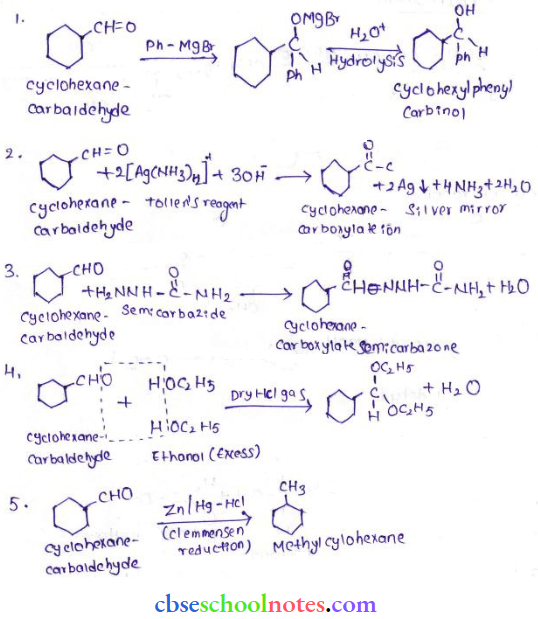
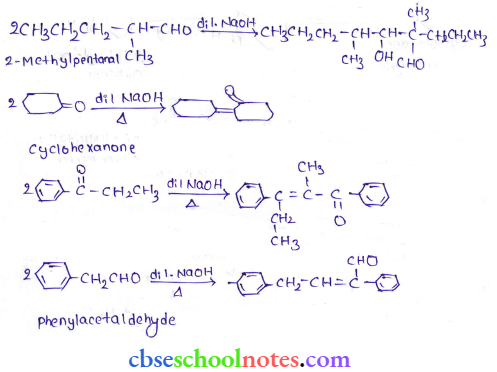
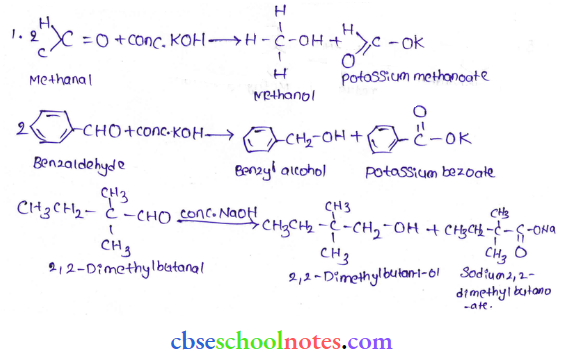

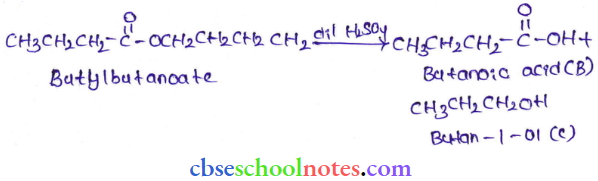
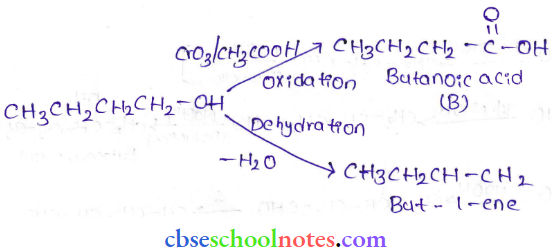
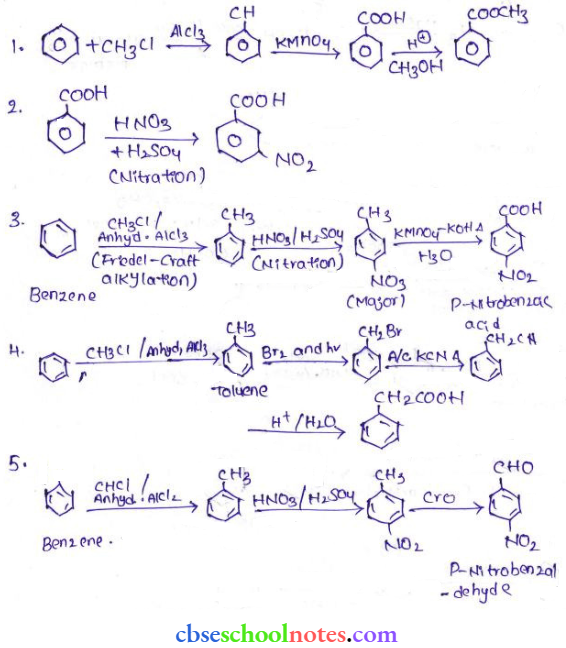
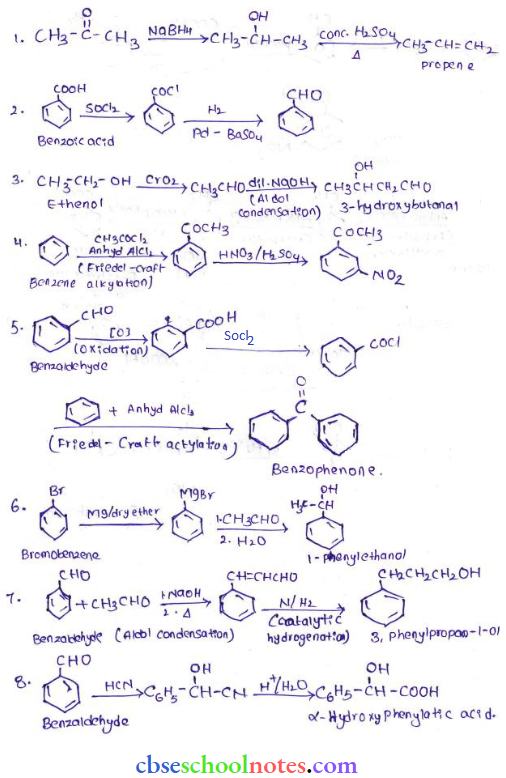
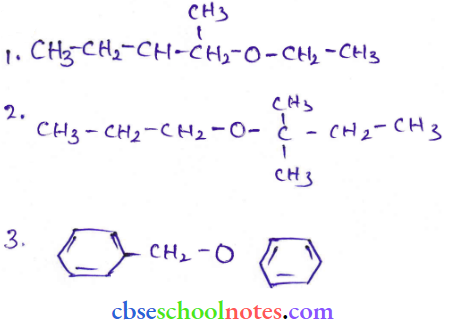
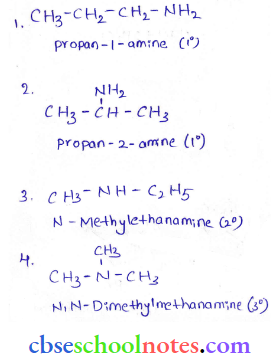
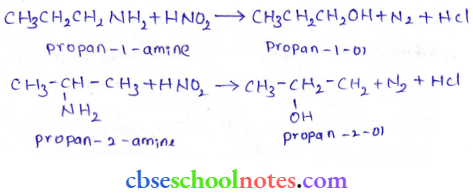
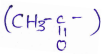 in an organic compound.
in an organic compound.