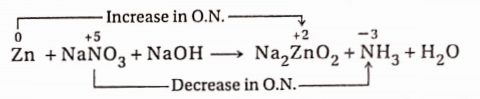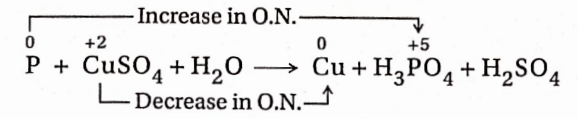Balancing Of Chemical Equations Involving Redox Reactions
Redox reaction can be balanced with the help of two methods. These are the
- Ion-electron method
- Oxidation number method.
Ion-electron method
Jade and Lamer in 1927 introduced this method. In the ion-electron method, only the molecules and ions which participate in the chemical reaction are shown.
In balancing redox reactions by this method the following steps are followed:
- The reaction is written in ionic form.
- The reaction is divided into two half-reactions with the help of ions and electrons. One half-reaction is for oxidation reaction and the other half-reaction is for reduction reaction.
- While writing the oxidation reaction, the reducing agent and the oxidised substance are written respectively on the left and right of an arrow signing are written respectively on the left and right of the arrow sign
- To denote the loss of electrons in oxidation half-reaction, the number of electrons (s) is written on the right of the arrow sign (→). While writing the reduction half-reaction, the number of electrons (s) gained is written on the left arrow sign ( →).
Thus, the oxidation half-reaction is:
Reducing agent – Oxidised substance +ne [where n = no. of electron (s) lost in oxidation reaction] Thus reduction half-reaction is Oxidising agent + ne Reduced substance [where n = no. of electron(s) gained reduction reaction] 2Cr3+
Then each half-reaction is balanced according to the following steps:
In each of the half-reactions, the number of atoms other than H and O -atoms on both sides ofthe arrow sign is balanced.
If a reaction takes place in an acidic medium, for balancing the number of H and O-atoms on both sides of the arrow sign, H2O or H+ is used. First, oxygen atoms are balanced by adding H2O molecules to the side that needs O-atoms.
Then to balance the number of H-atoms, two H+ ions (2H+) for each molecule of water are added to the opposite side (i.e., the side deficient in hydrogen atoms) of the reaction occurs in an alkaline medium, for balancing the H and O -atoms, H2O or OH– ion is used.
- Each excess oxygen atom on one side of the arrow sign is balanced by adding one water molecule to the same side and two ions to the other side.
- If the hydrogen atom is still not balanced, it is then balanced by adding one OH– for every excess hydrogen atom on the side of the hydrogen atoms and one water molecule on the other side of the arrow sign in a half-reaction, both H+ and OH– ions cannot participate.
- The charge on both sides of each half-reaction is balanced. This is done by adding an electron to that side which is a deficient negative charge.
- To equalise the number of electrons of the two half-reactions, any one of the reactions or both reactions should be multiplied by suitable integers.
- Now, the two half-reactions thus obtained are added. Cancelling the common term(s) on both sides, the balanced equation is obtained.
Examples:
1. In the presence of H2SO4, potassium dichromate (K2Cr2O2) and ferrous sulphate (FeSO4) react together to produce ferric sulphate [Fe2(SO4)3] and chromic sulphate [Cr2(SO4)3].
Reaction:
K2Cr2O7 + FeSO4 + H2SO4→ K2SO4 + Cr2(SO4)3 + Fe2(SO4)3 + H2O
The reaction can be expressed in ionic form as:
Cr3+ + Fe3+ + H2O
Oxidation half-reaction: Fe2+→Fe3++ e ……………………(1)
Reduction half-reaction: Cr2O72-+ Cr3+ ……………………(2)
1. Balancing the Cr -atom: Cr2O72-– Cr3+ 7H2O
2.To equalise the number of O -atoms on both sides, 7 water molecules are to be added to the right side. 2Cr3+ + 7H2O
⇒ \(\mathrm{Cr}_2 \mathrm{O}_7^{2-} \longrightarrow 2 \mathrm{Cr}^{3+}+7 \mathrm{H}_2 \mathrm{O}\)
∴ One water molecule is required for each O-atom.
3. To balance H-atoms on both sides, 14H+ ions are to be added to the left side.
⇒ \(\mathrm{Cr}_2 \mathrm{O}_7^{2-}+14 \mathrm{H}^{+} \longrightarrow 2 \mathrm{Cr}^{3+}+7 \mathrm{H}_2 \mathrm{O}\)
∴ 2H+ ions are required for each water molecule.
4. For equalising the charge on both sides, 6 electrons are to be added to the left side.
⇒ \(\mathrm{Cr}_7 \mathrm{O}_7^{2-}+14 \mathrm{H}^{+}+6 e \longrightarrow 2 \mathrm{Cr}^{3+}+7 \mathrm{H}_2 \mathrm{O}\)
Now, for balancing the number of electrons in oxidation and reduction half-reactions, the balanced oxidation half-reaction is multiplied by 6 and the balanced reduction half-reaction by 1. Then these two equations are added.

This balanced equation has been expressed in ionic form. This equation can be represented in molecular form as:
6FeSO4 + K2Cr2O7 + 7H2SO4 → 3Fe2(SO4)3 + Cr2(SO4)3+ K2SO4+ 7H2O
∴ For 2H+ ions, one H2SO4 molecule is required
2. In presence of H2SO4, KMnO4 and FeSO4 react together to produce MnSO4 and Fe2(SO4)3.
Reaction: KMnO4 + FeSO4 + H2SO4 → K2SO4 + MnSO4+ Fe2(SO4)3 + H2O
The equation can be expressed in ionic form as:
MnO4 + Fe2+ + H+→-Mn2+ + Fe3+ + H2O
Oxidation half-reaction: Fe2+ — Fe3+ + e ………………………..(1)
Reduction half-reaction: MnO4– + 8H+ + 5e → Mn2+ + 4H2O………………………..(2)
To balance the number of electrons lost in the oxidation half-reaction, the oxidation half-reaction is multiplied by 5 and then the two reactions are added.

As one Fe2(SO4)3 molecule contains two Fe -atoms, the equation is multiplied by 2
10Fe2+ + MnO4– + 16H+ →10Fe3+ + 2Mn2+ + 8H2O
This is the balanced equation in ionic form. This equation when expressed in molecular form becomes
10FeSO4 + 2KMnO4+ 8H2SO4 → 5Fe2(SO4)3 + 2MnSO4 + 8H2O
Equalising the number of atoms of different elements and the sulphate radicals we get,
10FeSO4+ 2KMnO4 + 8H2SO4 → 5Fe2(SO4)3 + 2MnSO4 + K2SO4 + 8H2O
This is a balanced equation of the given reaction in molecular form.
3. In the reaction between K2Cr2O7 acidified with dilute H2SO4 and KI, Cr2(SO4)3 and I2 are formed.
K2Cr2O7+KI + H2SO4 → K2SO4 + Cr2(SO4)3 + I2 + H2O
The equation can be expressed in ionic form as— Cr2O72-+I– + H+ — Cr3+ + I2 + H2O
Oxidation half-reaction: 2I–→ I2 + 2e……………..(1)
Reduction half-reaction: Cr2O7 2-+ I–+14H+ + 6e — 2Cr3+ + 7H2O ……………………….(2)
To balance the electrons, equation (1) is multiplied by 3 and added to equation (2). Thus the equation stands as—

This is the balanced equation of the reaction in ionic form. The above ionic reaction can be expressed in molecular form as follows
6KI + K2Cr2O7 + 7H2SO4→3I2 + Cr2(SO4)3 + 7H2O
Equalising the number of atoms of potassium and sulphate radical on the left and right sides, we have,
6KI + K2Cr2O7+ 7H2SO4 → 3I2 + Cr2(SO4)3 + 4K2SO4 + 7H2O
4. In the reaction between KMnO4, acidified with dilute H2SO4 and oxalic acid (H2C2O4), MnSO4 and CO2 were produced.
Reaction: KMnO4 + H2C2O4 + H2SO4 → K2SO4 + MnSO4 + CO2 + H2O
Oxidation half-reaction: C2O4→ 2CO42-+ 2e ……………………..(1)
Reduction half-reaction: MnO4– +8H++ 5e → Mn2++ 4H2O……………………..(2)
Now, multiplying equation (1) by 5 and equation (2) by 2 and then adding them, we get,
5C2O4 2-+ 2MnO4 + 16H++10CO2 + 2Mn2+ + 8H2O
This Is the balanced equation of the given reaction in molecular form.
5H2C2O4 + 2KMnO4+ 3H2SO4→ 10CO2 + Mn2+ +8H2O
Equalising the number of atoms of potassium and sulphate radical we get
5H2C2O4 + 2KMnO4 + 3H2SO4 → 10CO2 + 2MnSO4 + K2SO4 + 8H2O
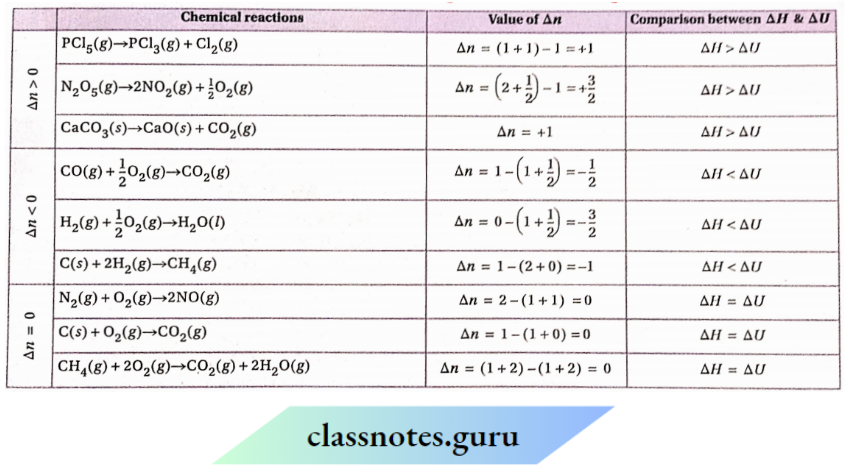
5. In NaOH solution, Zn reacts with NaNO3 to yield Na2ZnO2, NH3 and H2O.
Reaction:
Zn + NaNO3 + NaOH — Na2ZnO2 + NH3 + H2O The equation can be expressed in ionic form as—
Zn + NO– + OH–→ ZnO2– →+ NH3 +H2O
Oxidation half-reaction: Zn + 4OH–→ ZnO2– + 2H2O + 2C ……………………..(1)
Reduction half-reaction: NO3 + 6H2O + 8C — NH3 + 9OH– ……………………..(2)
Now multiplying equation (1) by 4 and then adding to equation (2), we get,
4Zn + 16OH- + NO, + 6H2O→ 4ZnO2 + NH3 + 90H- + 8H2O
Or, 4Zn + 7OH–+ NO–3→ 4ZnO2– + NH3 + 2H2O
It is the balanced equation of the reaction in ionic form. Expressing the above equation in molecular form
4Zn + 7NaOH + NaNO3→ 4Na2ZnO2 + NH3 + 2H2O
It is the molecular form of the balanced equation of the reaction.
6. In the presence of HNO3, sodium bismuthatic (NaHO3) reacts with Mn(NO3)2 to produce coloured sodium permanganate (NaMnO4) and itself gets reduced to bismuth nitrate.
Reaction: NaBIO3 + Mn(NO3) + UNO2 →NaMnO4 + Bi(NO3) + H2O The equation can be expressed in ionic form as— BIO2 + Mn2+ → Bl3+ + MnO4 + H2O
Oxidation half-reaction: Mn2+ + 4H2O → MnO4–+8H+→+5e ……………………(1)
Reduction half-reaction: BiO3 + 6H+ + 2e — Bi3+ + 3H2O…………………….(2)
Multiplying equation (1) by 2 and equation (2) by 5 and then adding them we get—
2Mn2+ + 8H2O + 5BiO3 – +30H+→ 2MnO4– + 16H+ + 5Bi3+ +15H2O
2Mn2+ + 5BiO3 + 14H+→ 5Bi3++ + 2MnO4– +7HO
This is the balanced ionic equation of the reaction. The equation in the molecular form stands as—
2Mn(NO3)2 + 5NaBiO3+ 14HNO3 → 5Bi(NO3)3 + 2NaMnO4 + 7H2O
Ionic reaction: IO3– + I–+ H+→ I2 + H2O
Oxidation half-reaction: 2I– → I2 + 2e ……………………….(1)
Reduction half-reaction: 2 IO3– + 12H++ 10 e → I2 + 6H2O ……………………….(2)
Multiplying equation (1) by 5 and then adding to equation (2) we get,
10I– + 2IO3–+ 12H+ → 6I2+ 6H2O or, 5I– + 1O3–+ 6H+ — 3I2 + 3H2O
This is the balanced ionic equation of the reaction.
Oxidation number method
In any redox reaction, the increase in the oxidation number of some of the atoms is balanced by the decrease in the oxidation number of some other atoms.
The steps which are to be followed while balancing the oxidation-reduction equation by this method are given below— After identifying the oxidant and reductant, the skeleton equation for the reaction is written.
- The elements of the reactants and the products changing oxidation number are identified and the oxidation number of the concerned atoms is mentioned.
- The reactant in which the element undergoes a decrease in oxidation number is the oxidant, while the reactant in which the element undergoes an increase in oxidation number is the reductant.
- As oxidation and reduction are complementary to each other, die increase and decrease in oxidation numbers should necessarily be equal, For this reason, the respective formulae of the oxidants and reductants are multiplied by a possible suitable integer so that the changes in oxidation numbers arc equalised.
- For balancing the equation, it may sometimes be necessary to multiply the formula of other substances participating in the reaction by a suitable integer.
- If the reactions are carried out in an acidic medium, then, to balance the number of O -atoms, one molecule of O–is added for each O -atom to the side of the equation deficient in oxygen.
To balance the number of -atoms, H+ ions are added to the side deficient in hydrogen.
In case of a reaction occurring in an alkaline medium, to balance the number of O -atoms on both sides of the equations, for each O -atom one molecule of water is added to the side deficient in O -atoms and to the opposite side two OH- ions for each water molecule are added.
Again for balancing the number of FI– atoms on both sides of the equation, for each 2 -atom one OH– ion is added to the side which contains excess 2 -atoms and the same number of FI2O molecules are added to the other side.
Example
1. Copper dissolves in concentrated HNO3 to form Cu(NO3)2, NO2 and H2O
Reaction:

In the given reaction, the increase in oxidation number of Cu -atom =(+2)-0 = 2 unit (oxidation) and the decrease in oxidation number of N -atom =(+5)-(+4) = 1 unit (reduction).
To nullify the effect of increase and decrease in the oxidation numbers, the ratio of the number of Cu -atoms and Cu(NO3)2 molecules in the reaction should be 1:2. So the equation may be written as
Cu + 2HNO3 → Cu(NO3)2 + 2NO2 + H2O
Now, to produce one molecule of Cu(NO3)2 two NO2 radicals i.e. two molecules of UNO2 are required. Hence in the reaction further addition of two molecules of HNO3 is necessary. So the balanced equation is expressed as
Cu + 4HNO3→ Cu(NO3)2 + 2NO2 + 2H2O
Now, to produce one molecule of Cu(NO3)2, two NO3 radicals i.e. two molecules of UNO2 are required.
Hence in the reaction further addition of two molecules of HNO2 is necessary. So the balanced equation is expressed as
Cu+4HNO3 Cu(NO3)→ 2+2NO2+2H2O
When H2S gas is passed through chlorine water H2SO4 is produced.
Reaction:

In this reaction, an increase in the oxidation number of S = (+6) — (— 2) = 2 units (oxidation) and a decrease In the oxidation number of Cl = 0 – (— 1 ) I unit (reduction). So decrease In oxidation number for two (‘,1 -atoms or I molecule of Cl2 -2 unit.
To neutralise the effect of Increase and decrease In oxidation number in the given equation, the number of molecules of H2S and Cl2 should be in the ratio of 2: i.e., 1: <1.
Therefore, the equation becomes
H2S+4CI2+HCl+H2SO4
Balancing the number of 11 and O -atoms on both sides gives the balanced equation —
3. NH3 gas when passed over heated Cut) produces Cu, N2 and H2O.

In this reaction, increase In oxidation number of N=0-(-3) = 3 unit (oxidation) and decrease In oxidation number of Cu =(+2)-0 = 2 unit (reduction). As, in a redox reaction, the total increase in oxidation number is equal to the total decrease In oxidation number, the number of molecules of CuO and NH3 in the reaction should be in the ratio of 3:2. Hence, the balanced equation will be—
3CuO+2NH3→3Cu+N2+3H2O
4. In the reaction between KMnO4 and H2O2, the products obtained were K2SO2 MnSO2, H2O And O2.
Reaction:

In this reaction increases in oxdination number of O = 0-(-1)=1 (oxidaxtion) and dexrease in oxidation number of MN= (+7)-(+20)
= 5 unit (reduction).
The total increase in the oxidation number of two 0 -atoms presents one molecule of H2O2
To balance the decrease and increase in oxidation numbers, the ratio of the number of KMnO2 and H2O, molecules in the equation for the reaction will be 2:5.
Again from 2 molecules of KMnO4 and 5 molecules of H2O2, 2 molecules of MnSO2 and 5 molecules of O2 are produced respectively. Thus the equation becomes—
2KMnO4 + 5H2O2+ H2SO4→ K2SO4+ 2MnSO4 + 5O2 + H2O
Again, for the formation of 1 molecule of K2SO4 and 2 molecules of MnSO4, three SO4– radicals are required and hence three H2SO4 molecules are necessary on the left-hand side. Besides this, the total number of H-atoms in 5 molecules of H2O2 and 3 molecules of H2SO4 = 16.
These H-atoms produce water molecules. Therefore, 8 molecules of H2O are to be placed on the right-hand side. So the balanced equation will be—
2KMnO4 + 5H2O2 + 3H2SO4 → K2SO4 + 2MnSO4 + 5O2+ 8H2O
5. White phosphorus and concentrated NaOH react together to yield NaH2PO2 and PH3. Reaction

The increase in oxidation number of P [P to NaH2PO2 ] = +1- 0 = 1 unit (oxidation). The decrease in oxidation number of P [P to PH3] = 0-(-3) = 3 unit (reduction). To balance the increase and decrease in oxidation number, three P atoms for oxidation and one P -atom for reduction are required. Thus four P -atoms are necessary.
Now, in the oxidation of P, NaH2PO2 and its reduction, PH3 are formed. So the oxidation of three P atoms forms 3 molecules of NaH2PO9 and for this, three NaOH molecules are required. Again 1 atom of P reduction produces 1 molecule of PH3. So the equation will be
P4+3NaOH + H2O→ 3NaH2PO2 + PH3
On the right side of the equation, there are 6 oxygen atoms, out of which 3 atoms will come from 3 molecules of NaOH and for the rest three atoms, 3 molecules of H2O will be necessary. Hence, the balanced equation will be
P4+ 3NaOH + H2O → 3NaH2PO2 + PH3
6. In NaOH solution, Zn reacts with NaNO3 to yield Na2ZnO2, NH3 and H2O.
Reaction:
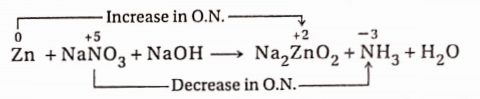
In the reaction, an increase in the oxidation number of Zn =(+2) -0 = 2 unit (oxidation) and a decrease in the oxidation number of N =(+5)-(-3) = 8 unit (reduction). As the increase and decrease in oxidation number in the reaction must be equal, the number of Zn -atoms and the number of molecules of NaNO3 should be in the ratio of 4:1. Now, 1 molecule of NaNO3 and 4 atoms of Zn produce 1 molecule of NH3 and 4 molecules of Na2ZnO2 respectively. Therefore the reaction is—
4Zn + NaNO3 + NaOH →4Na2ZnO2 + NH3 + H2O
Again formation of 4 molecules of Na2ZnO2 requires 8 Na -atoms, out of which 1 atom is supplied by 1 molecule of NaNO3. Additional 7 Na -atoms come from NaOH on the left side. To balance H -atoms on both sides, 1 H2O molecule is to be placed on the right side. Thus the balanced equation will be —
4Zn + NaNO3 + 7NaOH →4Na2ZnO2 + NH3 + 2H2O
7. In the reaction between Cr2O3 and Na2O2, Na2CrO4 and NaOH are produced.
Reaction:
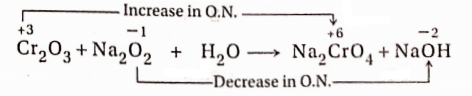
In this reaction, an increase in the oxidation number of Cr = (+6)- (+3) = 3 unit (oxidation) and a decrease in the oxidation number of O =(- 1 )-(- 2) = 1 unit (reduction). Thus a total increase in the oxidation number of two Cr -atoms = 3 × 2 = 6 units and the total decrease in the oxidation number of two O -atoms = 1× 2
= 2 units.
To balance the increase and decrease in oxidation number, the ratio of Cr2O3 and Na2O2 should be =1:3. Now 2 molecules of Na2CrO4 are produced from 1 molecule of Cr2O3. Hence the equation will be as follows—
Cr2O3+3Na2O2+H2O→ 2Na2CrO4+NaOH
If Na, H and O- atoms are balanced on both sides, the balanced equation will stand as
Cr2O3+ 3Na2O2 + H2O→ 2Na2CrO4 + 2NaOH
8. White phosphorus reacts with copper sulphate solution to produce Cu, H3PO4 and H2SO4.
Reaction:
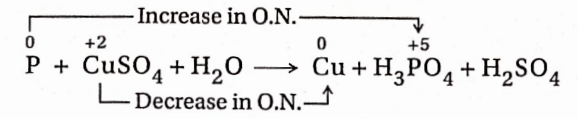
In this reaction, an increase in the oxidation number of P = (+5)-0 = 5 unit (oxidation) and a decrease in the oxidation number of Cu =(+2)-0 = 2 unit (reduction).
Since the increase and decrease in oxidation number must be equal, in the given reaction, the ratio of the number of atoms of P and the number of CuSO4 molecules should be in the ratio of 2: 5. Again 2 molecules of H3PO4 and five Cu -atoms will be produced respectively from two P atoms and five CuSO4 molecules. As a result, the equation becomes—
2P + 5CuSO4 + H2O→ 5Cu + 2H3PO4 + H2SO4
To balance the number of SO²‾4 radicals on both sides of the equation, 5 molecules of H2SO4 are to be added to the right-hand side ofthe equation.
2P + 5CuSO4 + H2O→5Cu + 2H3PO4 + 5H2SO4
Now, the total number of H-atoms present in 2 molecules of H3PO4 and 5 molecules of H2SO4 =16. So, for balancing the number of H-atoms, 8 water molecules are to be placed on the left-hand side. So, the balanced equation will be
2P + 5CuSO4 + 8H2O→5Cu + 2H3PO4 + 5H2SO4
9. Aluminium powder when boiled with caustic soda solution yields sodium aluminate and hydrogen gas.
Reaction:

Aluminium is oxidised in this reaction to produce sodium aluminate. On the other hand, the H -atoms of NaOH and H2O are reduced to produce H2. Therefore, the change in oxidation number in the reaction may be shown as follows—

The increase in oxidation number of A1 = (+3) -0 = 3 unit (oxidation), the decrease in oxidation number of 1 H atom of NaOH molecule \(=(+1)-\left(\frac{1}{2} \times 0\right)\) (reduction) and decrease in oxidation number of 2 H -atoms
of water molecule = 2 x (+1) -2×0 = 2 unit(reduction).
Hence, the total decrease in oxidation number for the Hatoms in 1 molecule of NaOH and 1 molecule of H2O =3 unit.
Since, in a chemical reaction, the increase and decrease in oxidation number are the same, the ratio of the number of A1 atoms, NaOH molecule and water molecules in the given reaction should be =1: 1: 1.
⇒ \(\mathrm{Al}+\mathrm{NaOH}+\mathrm{H}_2 \mathrm{O} \longrightarrow \mathrm{NaAlO}_2+\frac{1}{2} \mathrm{H}_2+\mathrm{H}_2\)
Hence, the given reaction may be represented as:
Now, to express the number of molecules of reactants and products in terms of whole numbers, both sides of the equation should be multiplied by 2.
So, the balanced equation will be as follows:
⇒ \(\mathrm{Al}+\mathrm{NaOH}+\mathrm{H}_2 \mathrm{O} \longrightarrow \mathrm{NaAlO}_2+\frac{1}{2} \mathrm{H}_2+\mathrm{H}_2\)
Determination of equivalent mass of an element or compound in disproportionation reaction:
If in oxidation and reduction reaction, the change in oxidation number of any element or an element of any compound participating in a disproportionation reaction be n1 and n2 respectively and M be the molecular mass of that element or compound, then the equivalent mass of that element or compound \(=\frac{M}{n_1}+\frac{M}{n_2}\)
In oxidation reaction (P4— change in oxidation number of each P -atom = 1 unit. So the total change in oxidation number of four P-atoms = 4 × 1 =4 units.
In the reduction reaction, (P4→PH3), the change in oxidation number of each P-atom is 3 units. So the total change in oxidation number of four P-atoms = 4×3 = 12 units.
Thus in this reaction, the equivalent mass of P4.
⇒ \(\frac{M}{4}+\frac{M}{12}=\frac{4 \times 31}{4}+\frac{4 \times 31}{12}=31+10.33 \text {= } 41.33\)
∴ The atomic mass of p = 31
Redox Titration
A process by which a standard solution of an oxidant (or a standard solution of a reductant) is completely reacted with a solution of an unknown concentration of a reductant (or with a solution of an unknown concentration of an oxidant) in the presence of a suitable indicator is called redox titration.
In a redox titration, an oxidant (or a reductant) reacts completely with an equivalent amount of a reductant (or an oxidant). Therefore, in a redox titration, the number of grams equivalent of oxidant = number of grams equivalent of reductant.
Types of redox titrations
1. Permanganometry titration
A titration in which KMnO4 solution is used as the standard solution. In this titration, no indicators are needed.
Example:
The amount of iron present in an acidic ferrous ion (Fe2+) solution can be estimated by titrating the solution with a standard solution of KMnO4.
Reaction:
MnO4 + 5Fe2+ + 8H+ → Mn2+ + 5Fe3+ + 4H2O …………………….(1)
Oxidation reaction: Fe2+→ Fe3+ + e
Reduction reaction: MnO4 –+ 8H+ + 5e → Mn2+ + 4H2O
So, in this reaction, the equivalent mass of Fe2+ — an atomic mass of Fe and the equivalent mass of KMnO4 \(=\frac{1}{5} \times\) Molecular or formula mass of KMnO4
According to the reaction (1), 1 mol of KMnO4 = 5 mol of Fe2+ ions or, 1000 mLof 1 mol of KMnO4 solution = 5 × 55.85g of Fe2+ ions or, 1 mLof l(M) KMnO4 solution = 0.2792g of Fe2+ ions mL of 5(N) KMnO4 solution = 0.2792g of Fe2+ ions. [In the given reaction, the normality of KMnO4 solution is five times its molarity.]
lmL of (N) KMnO4 solution = 0.05585g of Fe2+ ions
2. Dichromatometry titration
A ptration in which a standard solution of potassium dichromate (K2Cr2O7) is used.
In this titration, sodium or barium diphenylamine sulphonate or diphenylamine is used as an indicator.
Example:
The amount of iron present in an acidic ferrous ion (Fe2+) solution can be calculated by titrating the solution with a standard solution of K2Cr2O7.
Reaction:
Cr2O7+ 14H+ + 6Fe2+→ 2Cr3++ 6Fe2+ + H2O …………….(1)
In reaction (1), the equivalent mass of Fe2+ is equal to the atomic mass of Fe, & the equivalent mass of K2Cr2O7 is equal to one-sixth of its molecular or formula mass.
According to the reaction (1), 1 mol of K2Cr2O7 H 6mol of Fe2+ ions
Or, 1000mL of 1M K2Cr2O7 = 6 × 55.85 g of Fe2+ ions
Or, lmLof1M K2Cr2O7 solution s 0.3351g of Fe2+ ions
Or, lmL of 6N K2Cr2O7 solution s 0.3351g of Fe2+ ions
[In the given reaction, the normality of K2Cr2O7 solution is six times its molarity.]
lmL of IN K2Cr2O7 solution = 0.05585g of Fe2+ ions
3. Iodometry titration
In this titration, KI in excess is added to a neutral or an acidic solution of an oxidant. Consequently, the oxidant quantitatively oxidises I– ions (reductant), to form I2. The liberated I2 is then titrated with a standard Na2S2O3 solution using starch as an indicator.
The amount of liberated iodine is calculated from the volume of standard Na2S2O3 solution consumed in one titration. After the amount of liberated iodine is known, one can calculate the amount of oxidant by using the balanced chemical equation for the reaction of oxidant with iodine.
Example:
Iodometric titration is often used for quantitative estimation of Cu2+ ions. The addition of excess KI to a neutral or an acidic solution of Cu2+ ions results in oxidation of 1 to I2 and reduction of Cu2+ to Cu+.
So, in the reaction(l), the equivalent mass of \(\mathrm{Cu}^{2+}=\frac{2 \times \text { atomic mass of } \mathrm{Cu}}{2}=\text { atomic mass of } \mathrm{Cu}\)
The reaction of I2 with Na2S2O3 is:
⇒ \(2 \mathrm{~S}_2 \mathrm{O}_3^{2-}+\mathrm{I}_2 \rightarrow \mathrm{S}_4 \mathrm{O}_6^{2-}+2 \mathrm{I}^{-}\)
In this reaction, Oxidation reaction:
⇒ \(2 \mathrm{~S}_2 \mathrm{O}_3^{2-} \rightarrow \mathrm{S}_4 \mathrm{O}_6^{2-}+2 e\)
Reduction reaction: I2 + 2e → 2I–
Therefore, the equivalent mass of Na2S2O3
⇒ \(\frac{2 \times \text { molecular or formula mass of } \mathrm{Na}_2 \mathrm{~S}_2 \mathrm{O}_3}{2}\)
= molecular or formula mass of Na2S2O3
Equivalent mass of I2 \(=\frac{\text { molecular mass of } \mathrm{I}_2}{2}=\text { atomic mass of } \mathrm{I}\)
According to the reactions (1) and (2), 2mol of Cu2+ = 1 mol of I2 and 2 mol of Na2S2O3 s 1 mol of I2
Therefore, 2mol of Na2S2O3= 2 mol of Cu2+ or, 1 mol of Na2S2O3 = l mol of Cu2+ = 63.5g of Cu2+ or, l mol of lM Na2S2O3 solution = 63.5 g of Cu2+ or, 1 mol of IN Na2S2O3 solution = 63.5gof Cu2+ [As in the reaction of Na2S2O3 with I2, the equivalent mass of Na2S2O3 is equal to its molecular mass].