D And F Block
Question 1. The silver atom has filled d orbitals (4d ) in its ground state. How can you say that it is a transition element?
Answer: Ag has a filled 4d orbital (4d10 5s1) in its ground state. Now, silver displays two oxidation states (+1 and +2). In the +1 oxidation state, an electron is removed from the s-orbital. However, in the +2 oxidation state, an electron is removed from the d-orbital.
Thus, the d-orbital now becomes incomplete (4d9). Hence, it is a transition element.
Question 2. In the series Sc (Z = 21) to Zn (Z = 30), the enthalpy of atomization of zinc is the lowest, i.e., 126 kJ mol-1Why?
Answer: The extent of metallic bonding a clement undergoes decides the enthalpy of atomization.
- The more extensive the metallic bonding of an element, the more will be its enthalpy of atomization. In all transition metals (except Zn, electronic configuration: 3d10 4s2), some unpaired electrons account for their stronger metallic bonding.
- Due to the absence of these unpaired electrons, the inter-atomic electronic bonding is the weakest in Zn and as a result, it has the least enthalpy of atomization.
Question 3. Which of the 3d scries of the transition metals exhibits the largest number of oxidation states and why?
Answer: Mn (Z = 25) = 3d5 4s2‘ Mm has the maximum number of unpaired electrons present in the d-subshell (5 electrons). Hence. Mn exhibits the largest number of oxidation states, ranging from +2 to +7.
Question 4. The E0(M2+/M) value for copper is positive (+0.34V). What is possibly the reason for this? (Hint: consider its high energy and low energy)
Answer: The E0(M2+/M) value of a metal depends on the energy changes involved in the following:
1. Sublimation: The energy required for converting one mole of an atom from the solid state to the gaseous state.
M(s) → M(g) ΔsH(Sublimation energy)
2. Ionization: The energy required to take out electrons from one mole of atoms in the gaseous state.
M(g) → M2+(g) ΔiH(Ionization energy)
3. Hydration: The energy released when one mole of ions is hydrated.
M2+(g)→ M2+(aq) ΔhydH(Hydration energy)
Now. copper has a high energy of atomization and low hydration energy.
Hence, the Eθ(M2+/M) value for copper is positive.
Read and Learn More Class 12 Chemistry with Answers Chapter Wise
Question 5. How would you account for the irregular variation of ionization enthalpies (first and second) in the first series of (the transition elements?
Answer: Ionization enthalpies are found to increase in the given series due to a continuous filling of the inner d-orbitals.
- The irregular variations of ionization enthalpies can be attributed to the extra stability of configurations such as d0, d5, and d10. Since these states arc exceptionally stable, their ionization enthalpies are very high.
- In the case of first ionization energy. Cr has low ionization energy. This is because after losing one electron, it attains the stable configuration (3d5 ). On the other hand, Zn has exceptionally high first ionization energy as an electron has to be removed from stable and fully-filled orbitals (3d10 4s2).
- The second ionization energies are higher than the first since it becomes difficult to remove an electron when an electron has already been taken out.
- Also, elements like Cr and Cu have exceptionally high second ionization energies as after losing the first electron, they have attained the stable configuration (Cr+:3d5 and Cu+:3d10). Hence, taking out one electron more from this stable configuration will require a lot of energy.
Question 6. Why is the highest oxidation state of a metal exhibited in its oxide or fluoride only?
Answer: Both oxide and fluoride ions are highly electronegative and have a very small size. Due to these properties, they can oxidize the metal to its highest oxidation state.
Question 7. Which is a stronger reducing agent Cr2+ or Fe2+ and why?
Answer: The following reactions are involved when Cr2+ and Fe2+ act as reducing agents.
→ Cr Cr2+ 3+ Fe2+ → Fe3+
The value is -0.41 V and is +0.77 V. This means that Cr2+ can be easily oxidized to Cr3+, but Fe does not get oxidized to Fe3+ easily. Therefore. Cr2+ is a better-reducing agent than Fe3+.
Question 8. Calculate the ‘spin only’ magnetic moment of M2+(aq), ion (Z = 27).
Answer:
Z = 27
⇒ [ Ar] 3d7 4s2
M2+ = [Ar] 3d7
![]()
i.e., 3 unpaired electrons
.’. n = 3
⇒ \(\sqrt{n(n+2)}=\mu\)
⇒ \(\sqrt{3(3+2)}=\mu\)
⇒ \(\sqrt{15}=\mu\)
⇒ \(\mu \approx 4 B M\)
Question 9. Explain why the Cu+ ion is not stable in aqueous solutions.
Answer: In an aqueous medium, Cu2+ is more stable than Cu+. This is because although energy is required to remove one electron from Cu+ to Cu2+, the high hydration energy of Cu2+ compensates for it. Therefore. Cu+ ions in an aqueous solution are unstable. It is disproportionate to give Cu2+ and Cu.
Question 10. Actinoid contraction is greater from element to element than lanthanide contraction. Why?
Answer: In actinoids, 5f orbitals are filled. These 5f orbitals have a poorer shielding effect than 4f orbitals (in lanthanides).
Thus, the effective nuclear charge experienced by electrons in valence shells in the case of actinides is much more than that experienced by lanthanides. Hence, the size contraction in actinides is greater as compared to that in lanthanides.
Question 11. Write down the electronic configuration of:
- Cr3+
- Pm3+
- Cu+
- Ce4+
- Co2+
- Lu2+
- Mn2+
- Th4+
Answer:
- Cr3+: 1s2 2s2 2p6 3s2 3p6 3d3 Or, [AT]18 3d3
- Pm3+: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f4 Or, [Xe]54 4f4
- Cu+ : 1s2 2s2 2p6 3s2 3p6 3d10 Or, [Ar]18 3d10
- Ce4+ : 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4P6 4d10 5s2 5p6 Or, [Xe]54.
- Co2+: 1s2 2s2 2p6 3s2 3p6 3d7 Or, [Ar ]18 3d7
- Lu2+: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d1 Or, [Xe]54 4f14 5d1
- Mn2+: 1s2 2s2 2p6 3s2 3p6 3d5 Or, [Ar]18 3d5
- Th4+: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 4f14 5s2 5p6 5d10 6s2 6p6 Or, [Rn]86
Question 12. Why are Mn2+ compounds more stable than Fe2+ towards oxidation to their +3 state?
Answer: The electronic configuration of Mn2+ is [Ar]18 3d5. (half-filled stability) The electronic configuration of Fe2+ is [Ar]18 3d6. (After losing one electron gain half-filled stability).
Question 13. Explain briefly how the +2 state becomes more and more stable in the first half of the first-row transition elements with increasing atomic number.
Answer: The oxidation states displayed by the first half of the first row of the transition metals arc are given in the table below.
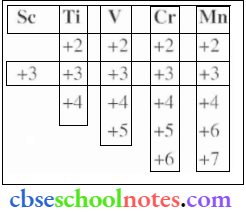
It can be easily observed that except Sc, all other metals display a +2 oxidation state. Also, on moving from Sc to Mn, the atomic number increases from 21 to 25. This means the number of electrons in the 3d-orbital also increases from 1 to 5.
- Sc (+2) = d1
- Ti (+2) = d2
- V (+2) = d3
- Cr (+2) = d4
- Mn (+2) = d5
+2 oxidation state is attained by the loss of the two 4s electrons by these metals. Since the number of d electrons in the (+2) state also increases from Tit+2) to Mn(+2), the stability of+2 state increases (as d-orbital is becoming more and more half-filled). Mn(+2) has d electrons (that is a half-filled d shell, which is highly stable).
Question 14. To what extent do the electronic configurations decide the stability of oxidation states in the first series of transition elements? Illustrate your answer with examples.
Answer: The elements in the first – half of the transition series exhibit many oxidation states with Mn exhibiting a maximum number of oxidation states (+2 to +7). The stability of the +2 oxidation state increases with the increase in atomic number. This happens as more electrons are getting filled in the d-orbital.
Question 15. What may be the stable oxidation state of the transition element with the following d electron configurations in the ground state of their atoms: 3d3, 3d5, 3d8 and 3d4?
Answer:
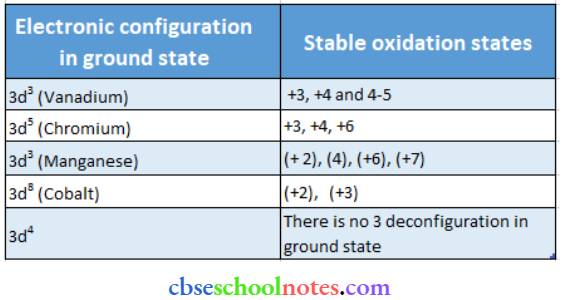
Question 16. Name the oximeter anions of (the first series of transition metals in which the metal exhibits the oxidation state equal to its group number.
Answer:
- Vanadate, VO–3 → The oxidation state of V is +5.
- Chromate. CrO2-4 → The oxidation state of Cr is +6
- Permanganate, MnO–4 → Oxidation state of Mn is +7.
Question 17. What is lanthanoid contraction? What are the consequences of lanthanoid contraction?
Answer: As we move along the lanthanoid series, the atomic number increases gradually by one.
- This means that the number of electrons and protons present in an atom also increases by one. As electrons are added to the same shell, the effective nuclear charge increases.
- This happens because the increase in nuclear attraction due to the addition of a proton is more pronounced than the increase in the inter-electronic repulsions due to the addition of an electron.
- Also, with the increase in atomic number, the number of electrons in the 4f orbital increases. The 4f electrons have a poor shielding effect. Therefore, the effective nuclear charge experienced by the outer electrons increases.
- Consequently, the attraction of the nucleus for the outermost electrons increases. This results in a steady decrease in the size of lanthanoids with an increase in the atomic number. This is termed as lanthanoid contraction.
Consequences of lanthanoid contraction
- There is a similarity in the properties of the second and third transition series.
- Separation of lanthanoids is possible due to lanthanide contraction.
- It is due to lanthanide contraction that there is variation in the basic strength of lanthanide hydroxides. (Basic strength decreases from La (OH)3 to Lu (OH)3)
Question 18. What are the characteristics of the transition elements and why are they called transition elements? Which of the d-block elements may not be regarded as the transition elements?
Answer:
Transition elements are those elements in which the atoms or ions (in a stable oxidation state) contain partially filled d-orbital. These elements lie in the d-block and show a transition of properties between the s-block and the p-block.
Therefore, these are called transition elements. Elements such as Zn, Cd and Hg cannot be classified as transition elements because these have filled the d-subshell.
Question 19. In what way is the electronic configuration of the transition elements different from that of the non-transition elements?
Answer:
Transition metals have a partially filled d-orbital. Therefore, the electronic configuration of transition elements is (n — 1 ) d1-10 ns0-2. The non-transition elements either do not have a d-orbital or have a filled d-orbital. therefore, the electronic configuration of non-transition elements is ns1-2 or ns2 np1-16.
Question 20. What are the different oxidation states exhibited by the lanthanoids?
Answer: In the lanthanide series. +3 oxidation stale is most common i.e., Ln (3) compounds are predominant. However. +2 and +4 oxidation states can also be found in the solution or in solid compounds.
Question 21. Explain giving reasons:
- Transition metals and many of their compounds show paramagnetic behaviour.
- The enthalpies of atomisation of the transition metals are high.
- The transition metals generally form coloured compounds.
- Transition metals and their many compounds act as good catalysts.
Answer:
- Transition metals show paramagnetic behaviour. Paramagnetism arises due to the presence of unpaired electrons with each electron having a magnetic moment associated with its spin angular momentum and orbital angular momentum.
- Transition elements have a high effective nuclear charge and a large number of valence electrons. Therefore, they form very strong metallic bonds. As a result, the enthalpy of atomization of transition metals is high.
- Most of the complexes of transition metals are coloured. This is because of the absorption of radiation from the visible light region to promote an electron from one of the d-orbitals to another. In the presence of ligands, the d-orbitals split up into two sets of orbitals having different energies.
- Therefore, the transition of electrons can take place from one set to another. The energy required for these transitions is quite small and falls in the visible region of radiation.
- The ions of transition metals absorb the radiation of a particular wavelength and the rest is reflected, imparting colour to the solution.
- The catalytic activity of the transition elements can be explained by two basic facts.
- Owing to their ability to show variable oxidation states and form complexes, transition metals form unstable intermediate compounds. Thus, they provide a new path with lower activation energy, Ea, for the reaction.
- Transition metals also provide a suitable surface for the reactions to occur.
Question 22. What are interstitial compounds? Why are such compounds well-known for transition metals?
Answer:
Transition metals are large and contain lots of interstitial sites. Transition elements can trap atoms of other elements (that have small atomic sizes), such as H, C, and N, in the interstitial sites of their crystal lattices. The resulting compounds are called interstitial compounds.
Question 23. How is the variability in oxidation states of transition metals different from that of the non-transition metals? Illustrate with examples.
Answer:
In transition elements, the oxidation state can vary from +1 to the highest oxidation state by removing all its valence electrons. Also, in transition elements, the oxidation stales differ by
- (Fe2+ and Fe3+: Cu+ and Cu2+). In non-transition elements, the oxidation states differ by
- For example. +2 and +4 or + 3 and +5. etc.
Question 24. Describe the preparation of potassium dichromate from iron chromite ore. What is the effect of increasing pH on a solution of potassium dichromate?
Answer:
Potassium dichromate is prepared from chromite ore (FeCr2O4) in the following steps.
Step (1): Preparation of sodium chromate
⇒ \(4 \mathrm{FeCrO}_4+16 \mathrm{NaOH}+7 \mathrm{O}_2 \longrightarrow 8 \mathrm{Na}_2 \mathrm{CrO}_4+2 \mathrm{FeO}_2+8 \mathrm{H}_2 \mathrm{O}\)
Step (2): Conversion of sodium chromate into sodium dichromate
⇒ \(2 \mathrm{NaCrO}_4+\text { conc. } \mathrm{H}_2 \mathrm{SO}_4 \longrightarrow \mathrm{Na}_2 \mathrm{Cr}_2 \mathrm{O}_7+\mathrm{Na}_2 \mathrm{SO}_4+\mathrm{H}_2 \mathrm{O}\)
Step (3): Conversion of sodium dichromate to potassium dichromate
⇒ \(\mathrm{Na}_2 \mathrm{Cr}_2 \mathrm{O}_7+2 \mathrm{KCl} \longrightarrow \mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7+2 \mathrm{NaCl}\)
Potassium dichromate being less soluble than sodium dichromate is obtained in the form of
orange-coloured crystals and can be removed by filtration.
The dichromate ion ( Cr2O2-4 ) exists in equilibrium with the chromate ( Cr2O24 ) ion at pH 4.
However, by changing the pH, they can be interconverted.
⇒ \(\underset{\text { Chromate ion(Yellow) }}{2 \mathrm{CrO}_4^{2-}}+2 \mathrm{H}^{+} \stackrel{\mathrm{PH}^{-4}}{\longrightarrow} \underset{\text { Dichromate ion(Orange) }}{\mathrm{Cr}_2 \mathrm{O}_7^{2-}}+\mathrm{H}_2 \mathrm{O}\)
On increasing pH, \(\mathrm{Cr}_2 \mathrm{O}_7^{2-}+2 \mathrm{OH}^{-} \longrightarrow 2 \mathrm{CrO}_4^{2-}+\mathrm{H}_2 \mathrm{O}\)
Question 25. Describe the oxidation action of potassium dichromate and write the ionic equations for its reaction with:
- Iodide
- Iron(2) solution and
- H2S
Answer:
K2Cr2O7 acts as a very strong oxidising agent in the acidic medium.
⇒ \(\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7+4 \mathrm{H}_2 \mathrm{SO}_4 \longrightarrow \mathrm{K}_2 \mathrm{SO}_4+\mathrm{Cr}_2\left(\mathrm{SO}_4\right)_3+4 \mathrm{H}_2 \mathrm{O}+3[\mathrm{O}]\)
K2Cr2O7 takes up electrons to gel reduced and acts as an oxidising agent. The reaction of K Cr 0_ with iodide ion, iron (2) solution and H S are given below.
1. K2Cr2O7 oxidizes iodide to iodine.
⇒ \(\mathrm{Cr}_2 \mathrm{O}_7^{2-}+14 \mathrm{H}^{+}+6 \mathrm{e} \longrightarrow 2 \mathrm{Cr}^{3+}+7 \mathrm{H}_2 \mathrm{O}\)
2I– → I2 + 2e– × 3
⇒ \(\mathrm{Cr}_2 \mathrm{O}_7^{2-}+6 \mathrm{I}^{-}+14 \mathrm{H}^{+} \longrightarrow 2 \mathrm{Cr}^{3+}+3 \mathrm{I}2+7 \mathrm{H}_2 \mathrm{O}\)
K2Cr2O7 oxidizes iron (2) solution to iron (3) solution i.e., ferrous ions to ferric ions.
⇒ \(\mathrm{Cr}_2 \mathrm{O}_7^{2-}+14 \mathrm{H}^{+}+6 \mathrm{e}^{-} \longrightarrow 2 \mathrm{Cr}^{3+}+7 \mathrm{H}_2 \mathrm{O}\)
Fe2+ → Fe3+ + e– × 6
⇒ \(\mathrm{Cr}_2 \mathrm{O}_7^{2-}+14 \mathrm{H}^{+}+6 \mathrm{Fe}^{2+} \longrightarrow 2 \mathrm{Cr}^{3+}+3 \mathrm{I}_2+7 \mathrm{H}_2 \mathrm{O}\)
K2Cr2O7 oxidizes H2S to sulphur.
⇒ \(\mathrm{Cr}_2 \mathrm{O}_7^{2-}+14 \mathrm{H}^{+}+6 \mathrm{e}^{-} \longrightarrow 2 \mathrm{Cr}^{3+}+7 \mathrm{H}_2 \mathrm{O}\)
H2S → S + 2H+ + 2e– × 3
⇒ \(\mathrm{Cr}_2 \mathrm{O}_7^{2-}+3 \mathrm{H}_2 \mathrm{~S}+8 \mathrm{H}^{+} \longrightarrow 2 \mathrm{Cr}^{3+}+3 \mathrm{~S}+7 \mathrm{H}_2 \mathrm{O}\)
Question 26. Describe the preparation of potassium permanganate. How does the acidified permanganate solution react with
- Iron(2) ions
- SO2
- Oxalic acid?
Write the ionic equations for the reactions.
Answer:
Potassium permanganate can be prepared from pyrolusite (MnO2). The ore is fused with KOH in the presence of either atmospheric oxygen or an oxidising agent, such as KNO3 or KCIO4 to give K2MnO4.
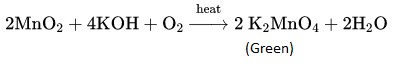
The green mass can be extracted with water and then oxidized either electrolytically or by passing chlorine/ozone into the solution.
Electrolytic oxidation
⇒ \(\mathrm{K}_2 \mathrm{MnO}_4 \longleftrightarrow 2 \mathrm{~K}^{+}+\mathrm{MnO}_4^{2-}\)
⇒ \(\mathrm{H}_2 \mathrm{O} \longleftrightarrow \mathrm{H}^{+}+\mathrm{OH}^{-}\)
At the anode, manganate ions are oxidized to permanganate ions.
⇒ \(\underset{\text { Green }}{\mathrm{MnO}_4^{2-}} \longleftrightarrow \underset{\text { Purple }}{\mathrm{MnO}_4^{-}}+\mathrm{e}^{-}\)
Oxidation by chlorine
⇒ \(2 \mathrm{~K}_2 \mathrm{MnO}_4+\mathrm{Cl}_2 \longrightarrow 2 \mathrm{KMnO}_4+2 \mathrm{KCl}\)
⇒ \(2 \mathrm{MnO}_4^{2-}+\mathrm{Cl}_2 \longrightarrow 2 \mathrm{MnO}_4^{-}+2 \mathrm{Cl}\)
Oxidation by ozone
⇒ \(2 \mathrm{~K}_2 \mathrm{MnO}_4+\mathrm{O}_3+\mathrm{H}_2 \mathrm{O} \longrightarrow 2 \mathrm{KMnO}_4+2 \mathrm{KOH}+\mathrm{O}_2\)
⇒ \(2 \mathrm{MnO}_4^2+\mathrm{O}_3+\mathrm{H}_2 \mathrm{O} \longrightarrow 2 \mathrm{MnO}_4^{2-}+2 \mathrm{OH}+\mathrm{O}_2\)
Acidified KMnO4 solution oxidizes Fe (2) ions to Fe (3) ions i.e., ferrous to ferric ions.
⇒ \(2 \mathrm{MnO}_4^2+\mathrm{O}_3+\mathrm{H}_2 \mathrm{O} \longrightarrow 2 \mathrm{MnO}_4^{2-}+2 \mathrm{OH}+\mathrm{O}_2\)
Fe2+ → Fe3+ + e– × 5
⇒ \(\mathrm{MnO}_{+}^{-}+5 \mathrm{Fe}^{2+}+8 \mathrm{H}^{+} \longrightarrow \mathrm{Mn}^{2+}+5 \mathrm{Fe}^{3+}+4 \mathrm{H}2 \mathrm{O}\)
Acidified potassium permanganate oxidizes SO2 to sulphuric acid.
⇒ \(\left.\mathrm{MnO}_4^{-}+6 \mathrm{H}^{+}+5 \mathrm{e} \longrightarrow \mathrm{Mn}^{2+}+3 \mathrm{H}_2 \mathrm{O}\right] \times 2\)
⇒ \(\left.2 \mathrm{H}_2 \mathrm{O}+2 \mathrm{SO}_2+\mathrm{O}_2 \longrightarrow 4 \mathrm{H}^{+}+2 \mathrm{SO}_4^{2-}+2 \mathrm{e}\right] \times 5\)
⇒ \(2 \mathrm{MnO}_4^{-}+10 \mathrm{SO}_2+5 \mathrm{O}_2+4 \mathrm{H}_2 \mathrm{O} \longrightarrow 2 \mathrm{Mn}^{2+}+10 \mathrm{SO}_4^{2-}+8 \mathrm{H}^{+}\)
Acidified potassium permanganate oxidizes oxalic acid to carbon dioxide.
⇒ \(\mathrm{MnO}_4+8 \mathrm{H}^{+}+5 \mathrm{e} \longrightarrow \mathrm{Mn}^{2+}+4 \mathrm{H}_2 \mathrm{O} \times 2\)
⇒ \(\left.\mathrm{C}_2 \mathrm{O}_4^2- \longrightarrow 2 \mathrm{CO}_2+2 \mathrm{c}\right] \times 5\)
⇒ \(2 \mathrm{MnO}_4^{-}+5 \mathrm{C}_2 \mathrm{O}_7^{2+}+16 \mathrm{H}^{+} \longrightarrow 2 \mathrm{Mn}^{2+}+10 \mathrm{CO}_2+8 \mathrm{H}_2 \mathrm{O}\)
Question 27. For M2+/M and M3+/M2++ systems, the E– values for some metals are as follows:
- Cr2+ /Cr – 0.9 V
- Cr3/Cr2+ – 0.4 V
- Mn2+/Mn -1.2V
- Mn3+/Mn2+ +1.5V
- Fe2+/Fe – 0.4 V
- Fe3+/Fe2+ + 0.8V
Use this data to comment upon:
- The stability of Fe3+ in acid solution as compared to that of Cr3+ and Mn3+ and
- The ease with which iron can be oxidised as compared to a similar process for either chromium or manganese metal.
Answer:
- The E– value for Fe3+/Fe2+ is higher than that for Cr3+ /Cr2+ and lower than that for Mn3+/Mn2+ So. the reduction of Fe3+ to Fe2+ is easier than the reduction of Cr3+ to Cr2+ but not as easy as the reduction of Mn3+ to Mn2+.
- Hence, Fe3+ is more stable than Mn3+, but less stable than Cr3+. These metal ions can be arranged in the increasing order of their stability as Mn3+< Fe3+ < Cr3+
The reduction potentials for the given pairs increase in the following order.
Mu2+ /Mn < Cr2+ /Cr < Fe2+/Fe
So, the oxidation of Fe to Fe2+ is not as easy as the oxidation of Cr to Cr2+ and the oxidation of Mn to Mn2+. Thus these metals can be arranged in the increasing order of their ability to get oxidised: Fe < Cr < Mn
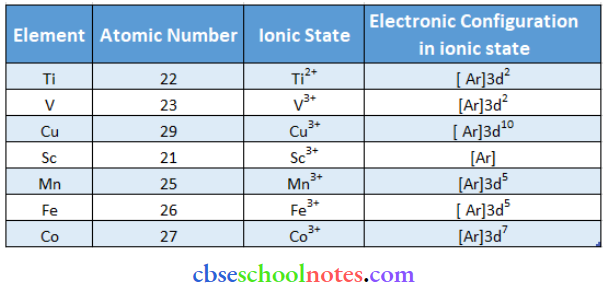
Question 28. Predict which of the following will be coloured in aqueous solution. Ti3+, V3+, Cu+, Sc3+, Mn2+, Fe3+ and Co2+. Give reasons for each.
Answer: Only the ions that have unpaired electrons in the el-orbital will be coloured. The ions in which the d orbital is empty or filled will be colourless.
Question 29. Compare the stability of the +2 oxidation state for the elements of the first transition series.
Answer:
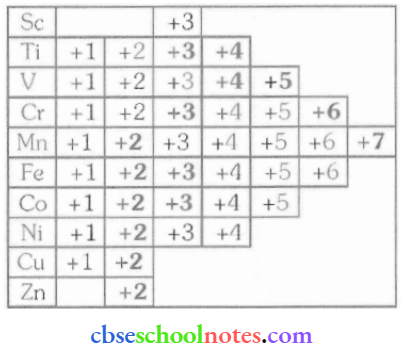
The relative stability of the +2 oxidation state increases on moving from top to bottom. This is because on moving from top to bottom, it becomes more and more difficult to remove the third electron from the d-orbital.
Question 30. Compare the chemistry of actinoids with that of the lanthanoids with special reference to:
- Electronic configuration
- Atomic and ionic sizes
- Oxidation state
- Chemical reactivity.
Answer:
- Electronic configuration: The general electronic configuration for lanthanoids is [Xe]54 4f1-145d0-1 6s2 and that for actinoids is [Rn]86 5f1-14 6d0-1 7s2
- Atomic and Ionic sizes: Similar to lanthanoids, actinoids also exhibit actinoid contraction (overall decrease in atomic and ionic radii). The contraction is greater due to the poor shielding effect of 5f orbitals.
- Oxidation states: The principal oxidation state of lanthanoids is (+3). However, sometimes we also encounter oxidation states of +2 and +4. This is because of the extra stability of fully-filled and half-filled orbitals. Actinoids exhibit a greater range of oxidation states. This is because the 5f, 6d, and 7s levels are of comparable energies.
- Chemical reactivity: In the lanthanide series, the earlier members of the series are more reactive. They have reactivity that is comparable to Ca. With an increase in the atomic number, the lanthanides start behaving similarly to Al. Actinoids, on the other hand- are highly reactive metals, especially when they are finely divided.
Question 31. How would you account for the following:
- Of the d4 species, Cr2+ is strongly reducing while manganese(3) is strongly oxidising.
- Cobalt(2) is stable in an aqueous solution but in the presence of complexing reagents, it is easily oxidised.
- The d1 configuration is very unstable in ions.
Answer:
- Cr2+ is strongly reducing in nature. It has a d4 configuration. While acting as a reducing agent, it gets oxidized to Cr3+ (electronic configuration, d3 ). This d3 configuration can be written as a t32g configuration, which is a more stable configuration. In the case of Mn3+ (d4), It acts as an oxidizing agent and gets reduced to Mn (d5). This has an exactly half-filled d-orbital and is highly stable.
- Co(2) is stable in aqueous solutions. However, in the presence of strong field complexing reagents, it is oxidized to Co(3). Although the 3rd ionization energy for Co is high, the higher amount of crystal field stabilization energy (CFSE) released in the presence of strong-field ligands overcomes this ionization energy.
- The ions in the d1 configuration tend to lose one more electron to get into the stable d0 configuration. Also, the hydration or lattice energy is more than sufficient to remove the only electron present in the cl -orbital of these ions. Therefore, they act as reducing agents.
Question 32. What is meant by ‘disproportionation’? Give two examples of disproportionation reactions in an aqueous solution.
Answer:
It is found that sometimes a relatively less stable oxidation state undergoes an oxidation-reduction reaction in which it is simultaneously oxidised and reduced. This is called disproportionation.
For example,
⇒ \(\underset{\mathrm{Cr}(\mathrm{5})}{3 \mathrm{CrO}_4^{3-}}+8 \mathrm{H}^{+} \longrightarrow \underset{\mathrm{Cr}(\mathrm{6})}{2 \mathrm{CrO}_{+}^{2-}}+\underset{\mathrm{Cr}(\mathrm{3})}{\mathrm{Cr}^{3+}}+4 \mathrm{H}_2 \mathrm{O}\)
Cr(5) is oxidized to Cr(6) and reduced to Crd(3)
⇒ \(\underset{\mathrm{Mn}(\mathrm{6})}{3 \mathrm{MnO}_4^{2-}}+4 \mathrm{H}^{+} \longrightarrow \underset{\mathrm{Mn}(\mathrm{7})}{2 \mathrm{MnO}_4^{-}}+\underset{\mathrm{Mn}(\mathrm{4})}{\mathrm{MnO}_2}+2 \mathrm{H}_2 \mathrm{O}\)
Mn (6) is oxidized to Mn (7) and reduced to Mn (4).
Question 33. Which metal in the first series of transition metals exhibits a +1 oxidation state most frequently and why?
Answer: In the first transition series, Cu exhibits a + 1 oxidation state very frequently. It is because Cu (+1) has an electronic configuration of [Ar] 3d10. The filled el-orbital makes it highly stable.
Question 34. Calculate the number of unpaired electrons in the following gaseous ions: Mn3+ \ Cr3+ \ V3+ and Ti3+. Which one of these is the most stable in aqueous solution?
Answer:
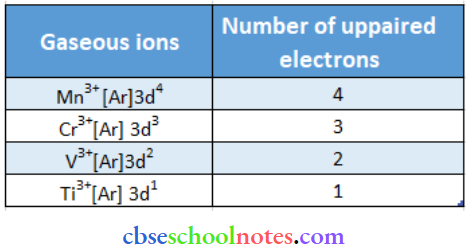
Cr3+ is the most stable in aqueous solutions owing to a t32g half-filled configuration.
Question 35. Give examples and suggest reasons for the following features of the transition metal chemistry:
- The lowest oxide of transition metal is basic, (and the highest is amphoteric/acidic.
- A transition metal exhibits the highest oxidation state in oxides and fluorides.
- The highest oxidation state is exhibited in oxoanions of a metal.
Answer:
- In the ease of a lower oxide of a transition metal, the metal atom has a low oxidation stale. This means that some of the valence electrons of the metal atom are not involved in bonding. As a result, it can donate electrons and behave as a base.
- On the other hand, in the case of a higher oxide of a transition metal, the metal atom has a high oxidation state. This means that the valence electrons are involved in bonding and so. they are unavailable. There is also a highly effective nuclear charge. As a result, it can accept electrons and behave as an acid.
- For example. Mn2O is basic and Mm27O7 is acidic.
- Oxygen and fluorine act as strong oxidising agents because of their high electronegatives and small sizes. Hence, they bring out the highest oxidation states from the transition metals.
- In other words, a transition metal exhibits higher oxidation states in oxides and fluorides. For example, in OsF6 and V2O5 the oxidation states of Os and V are +6 and +5 respectively.
- Oxygen is a strong oxidising agent due to its high electronegativity and small size. So. oxo-anions of metal have the highest oxidation state. For example, in MnO4– the oxidation state of Mn is +7.
Question 36. What are alloys? Name an important alloy which contains some of the lanthanoid metals Mention its uses.
Answer:
- An alloy is a solid of two or more elements in a metallic matrix. It can either be a partial solid solution or a complete solid solution. Alloys are usually found to possess different physical properties than those of the component elements.
- An important alloy of lanthanoids is Mischmetal. It contains lanthanoids (94-95%), iron (5%), and traces of S, C. Si. Ca and Al.
Uses:
- Mischmetal is used in cigarettes and gas lighters.
- It is used in flame-throwing tanks.
- It is used in tracer bullets and shells.
Question 37. What are inner transition elements? Decide which of the following atomic numbers are the atomic numbers of the inner transition elements: 29, 59, 74, 95, 102, 104.
Answer:
Inner transition elements are those elements in which the last electron enters the f-orbital. The elements in which the 4f and 5f orbitals are progressively filled are called f-block elements. Among the given atomic numbers, the atomic numbers of the inner transition elements are 59, 95 and 102.
Question 38. The chemistry of the actinoid elements is not as smooth as that of the Lanthanoids. Justify this statement by giving some examples of the oxidation state of these elements.
Answer:
- Lanthanoids primarily show three oxidation states (+2, +3, +4). Among these oxidation states. +3 state is the most common. Lanthanoids display a limited number of oxidation states because the energy difference between 4f, 5d and 6s orbitals is quite large.
- On the other hand, the energy difference between 5f, 6d, and 7s orbitals is very low. Hence aclinoids display a large number of oxidation states. For example, uranium and plutonium display +3. +4. +5. and +7. The most common oxidation state in the case of retinoids is also +3.
Question 39. Which are the last elements in the series of actinoids? Write the electronic configuration of this element. Comment on the possible oxidation state of this element.
Answer:
The last element in the actinoid series is lawrencium. Lr. Its atomic number is 103 and its electronic configuration is [Rn] 5f14 6d1 7s2. The most common oxidation state displayed by it is +3; because after losing 3 electrons it attains a stable f14 configuration.
Question 40. Use Hund’s rule to derive the electronic configuration of the Ce3+ ion and calculate its magnetic moment based on the ‘spin-only’ formula.
Answer:
Ce3+:1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f1
Magnetic moment can be calculated as:
⇒ \(\mu=\sqrt{n(n+2)}\)
Where, n = number of unpaired electrons.
In Ce3+, n = 1
Therefore, \(\mu=\sqrt{1(1+2)}=\sqrt{3}=1.732 \mathrm{BM}\)
Question 41. Name the members of the lanthanoid series which exhibit a +4 oxidation state and those which exhibit a +2 oxidation state. Try to correlate this type of behaviour with the electronic configuration of these elements.
Answer:
The lanthanoids that exhibit + 2 and +4 states are shown in the given table. The atomic numbers of these elements are given in parentheses.

- Ce after forming Ce4+ attains a stable electronic configuration of [Xe].
- Tb after forming Tb4+ attains a stable electronic configuration of [Xe] 4f7
- Eu after forming Eu2+ attains a stable electronic configuration of [Xe] 4f7
- Yb after forming Yb2+ attains a stable electronic configuration of [Xe] 4f14
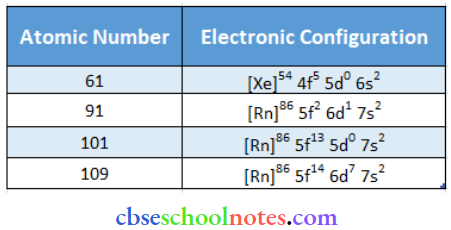
Question 42. Write the electronic configuration of the elements with the atomic numbers 61.91. 101. and 109.
Answer:
Question 43. Compare the general characteristics of the first series of the transition metals with those of the second and third series metals in the respective vertical columns. Give special emphasis on the following points:
- Electronic configurations.
- Oxidation states,
- Ionisation enthalpies, and
- Atomic sizes.
Answer:
1. In the 1st, 2nd and 3rd transition series, the 3d, 4d and 5d orbitals are respectively filled.
We know that elements in the same vertical column generally have similar electronic configurations.
In the first transition series, two elements show unusual electronic configurations:
- Cr(24) = 3d5 4s1
- Cu(29) = 3d10 4s1
Similarly, there are exceptions in the second transition series. These are:
- Nb(41) = 4d4 5s1
- Mo(42) = 4d5 5s1
- Tc (43) = 4d6 5s1
- Ru(44) = 4d7 5s1
- Rh(45) = 4d8 5 s1
- Pd(46) = 4d10 5s0
- Ag(47) = 4d10 5s1
There are some exceptions in the third transition series as well. These are:
- Pt(78) = 5d9 6s1
- Au(79) = 5d10 6s1
As a result of these exceptions, it happens many times that the electronic configurations of the elements present in the same group are dissimilar.
2. In each of the three transition series the number of oxidation states shown by the elements is the maximum in the middle and the minimum at the extreme ends.
- However, +2 and +3 oxidation states are quite stable for all elements present in the first transition series. All metals present in the first transition series form stable compounds in the +2 and +3 oxidation states.
- The stability of the +2 and +3 oxidation states decreases in the second and third transition series, wherein higher oxidation states are more important.
For example \(\left[\begin{array}{l}
\mathrm{2} \\
\mathrm{Fe}(\mathrm{CN})_6
\end{array}\right]^{4-} \cdot\left[\begin{array}{l}
\mathrm{3} \\
\mathrm{Co}\left(\mathrm{NH}_3\right)_6
\end{array}\right]^{3+} \cdot\left[\mathrm{Ti}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^3\) are stable complexes,
but no such complexes are known for the second and third transition series such as Mo, W, Rh, and In. They form complexes in which their oxidation states are high. For example WCI6, ReF7, RuO4 etc.
3. In each of the transition series, the first ionisation enthalpy increases from left to right. However, there are some exceptions.
- The first ionisation enthalpies of the third transition series are higher than those of the first and second transition series. This occurs due to the poor shielding effect of 4f electrons in the third transition series.
- Certain elements in the second transition series have higher first ionisation enthalpies than elements corresponding to the same vertical column in the first transition series.
- There are also elements in the 2′ transition series whose first ionisation enthalpies are lower than those of (the elements corresponding to the same vertical column in the 1 transition series.
4. Atomic si/.e generally decreases from left to right across a period. Now. among the three transition series, the atomic sizes of the elements in the second transition series are greater than those of the element corresponding to the same vertical column in the first transition series.
However, the atomic sizes of the elements in the third transition series are virtually the same as those of the corresponding members in the second transition series. This is due to lanthanoid contraction.
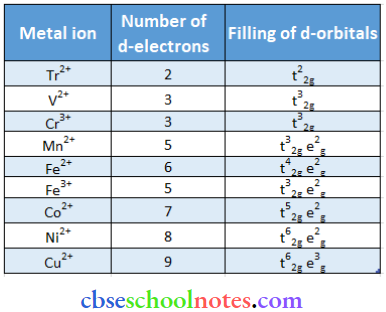
Question 44. Write down the number of 3d electrons in each of the following ions: Ti2+, V2+, Cr3+, Mn2+, Fe2+, Fe3+, Co2+, Ni 2+ and Cu2+ Indicate how would you expect the live 3d orbitals to be occupied for these hydrated ions (octahedral).
Answer:
Question 45. What can be inferred from the magnetic moment values of the following complex species?
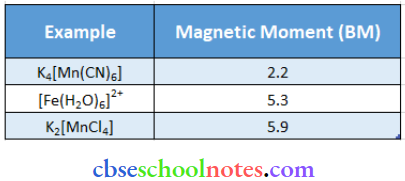
Answer:
Magnetic moment (μ) is given as μ = \(\sqrt{n(n+2)} B M\)
- For value n = 1, μ = \(\sqrt{1(1+2)}=\sqrt{3}=1.732 \mathrm{BM}\)
- For value n = 2, μ = \(\sqrt{2(2+2)}=\sqrt{8}=2.83 \mathrm{BM}\)
- For value n = 3, μ = \(\sqrt{3(3+2)}=\sqrt{15}=3.87 \mathrm{BM}\)
- For value n = 4, μ = \(\sqrt{4(4+2)}=\sqrt{24}=4.899 \mathrm{BM}\)
- For value n = 5, μ = \(\sqrt{5(5+2)}=\sqrt{35}=5.92 \mathrm{BM}\)
1. K4[Mn(CN)6]
For transition metals, the magnetic moment is calculated from the spin-only formula. Therefore.
⇒ \(\mu=\sqrt{n(n+2)}=2.2 \mathrm{BM}\)
We can see from the above calculation that the given value is closest to n = 1. Also, in this complex. Mn in in the 4-2 oxidation state. This means that Mn has 5 electrons in the d-orbital.
Hence, we can say that CN is a strong field ligand that causes the pairing of electrons.
2. [Fe(H2O)6]2+
⇒ \(\mu=\sqrt{\mathrm{n}(\mathrm{n}+2)}=5.3 \mathrm{BM}\)
We can see from the above calculation that the given value is closest to n = 4. Also, in this complex. Fe is in the 4-2 oxidation state. This means that Fe has 6 electrons in the d-orbital. Hence, we can say that H2O is a weak field ligand and does not cause the pairing of electrons.
3. K2[MnCI4]
⇒ \(\mu=\sqrt{n(n+2)}=5.9 B M\)
- We can see from the above calculation that the given value is closest to n = 5. Also, in this complex, Mn is in the 4-2 oxidation state. This means that Mn has 5 electrons in the d-orbital.
- Hence, we can say that Cl– is a weak field ligand and does not cause the pairing of electrons.
