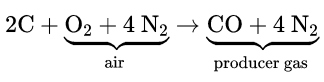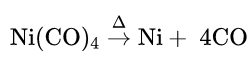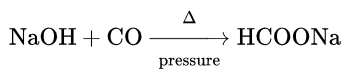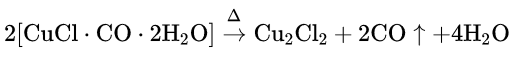Heat Of Reaction
Heat Of Reaction Definition
The amount of heat absorbed or evolved in a reaction when the stoichiometric number of moles of reactions indicated by the balanced chemical equation, is completely converted into products at given conditions is called the heat of reaction at that conditions.
At a particular temperature, the heat of a reaction depends on the conditions under which a reaction is occurring. Generally, chemical reactions are carried out either at constant pressure or at constant volume. Accordingly, the heat of reaction is of two types, namely Heat of reaction at constant volume and heat of reaction at constant pressure.
The heat of reaction at constant volume
The amount of heat absorbed or evolved in a reaction when the stoichiometric number of moles of reactants, indicated by the balanced chemical equation of the reaction, is completely converted into products at a fixed temperature and volume is called the heat of reaction at constant volume.
Explanation: Let us consider a chemical reaction that is occurring at constant temperature and volume.
⇒ \(a A+b B \rightarrow c C+d D\)
According to die first law of thermodynamics, if a reaction occurs at constant volume then the heat change (qv) is equal to the change in internal energy (AU) ofthe system (provided only pressure-volume work is performed), qv= ΔU
Therefore, for the reaction
⇒ \(q_V=\Delta U=\Sigma U_{\text {products }}-\Sigma U_{\text {reactants }}=\Sigma U_P-\Sigma U_R\)
where ΣUp = total internal energy of products and I UR = total internal energy of reactants.
∴ \(q_V=\Sigma U_p-\Sigma U_R=\left(c \bar{U}_C+d \bar{U}_D\right)-\left(a \bar{U}_A+b \bar{U}_B\right)\)
Where \(\bar{U}_A, \bar{U}_B, \bar{U}_C \text { and } \bar{U}_D\) die molar internal energies of A. B. Cand D, respectively. Thus, the heat of reaction for a reaction at constant temperature and volume is the difference between the total internal energy of tire products and the total internal energy of the reactants.
Example:
⇒ \(\mathrm{CH}_4(\mathrm{~g})+2 \mathrm{O}_2(\mathrm{~g}) \rightarrow \mathrm{CO}_2(\mathrm{~g})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{l})\)
For the above reaction, the heat of the reaction at a constant volume:
⇒ \(q_V=\left[\bar{U}\left(\mathrm{CO}_2, g\right)+2 \bar{U}\left(\mathrm{H}_2 \mathrm{O}, l\right)\right]-\left[\bar{U}\left(\mathrm{CH}_4, g\right)+2 \bar{U}\left(\mathrm{O}_2, g\right)\right]\)
Where
⇒ \(\bar{U}\left(\mathrm{CO}_2, \mathrm{~g}\right), \bar{U}\left(\mathrm{H}_2 \mathrm{O}, l\right), \bar{U}\left(\mathrm{CH}_4, g\right) \text { and } \bar{U}\left(\mathrm{O}_2, g\right)\)
Are die molar internal energies of CO,(g), H2O(I), CH4(g) respectively.
Heat of reaction at constant pressure:
The amount of heat absorbed or evolved in a reaction when the stoichiometric number of moles of reactants, indicated by a balanced chemical equation of the reaction, is completely converted into products at constant pressure, and temperature is called the heat of reaction at constant pressure.
The heat of reaction at constant pressure Explanation:
Let us consider a reaction that is occurring at constant temperature and pressure: aA + bB →+ cC + dD. According to the first law of thermodynamics, if a reaction occurs at constant pressure, then the heat change in the reaction is equal to the change in enthalpy of the system (provided only pressure-volume work is performed). Thus, qp = ΔH. Therefore, for the reaction,
⇒ \(q_P=\Delta H=\Sigma H_{\text {products }}-\Sigma H_{\text {reactants }}=\Sigma H_P-\Sigma H_R \text { ; }\)
where Hp = total enthalpy ofthe products and HR = total enthalpy of the reactants
∴ \(q_P=\Sigma H_P-\Sigma H_R=\left(c \bar{H}_C+d \bar{H}_D\right)-\left(a \bar{H}_A+b \bar{H}_B\right)\)
where, \(\bar{H}_A, \bar{H}_B, \bar{H}_C \text { and } \bar{H}_D\) are the molar enthalpies of A, B, C, and D, respectively.
Thus, for a reaction, the heat of the reaction at constant temperature and pressure is equal to the difference between the total enthalpy of products and the total enthalpy of reactants.
Example:
For the Reaction, \(2 \mathrm{H}_2(\mathrm{~g})+\mathrm{O}_2(\mathrm{~g}) \rightarrow 2 \mathrm{H}_2 \mathrm{O}(l)\) the heat ofreaction at constant pressure is given by;
⇒ \(q_P=2 \bar{H}\left(\mathrm{H}_2 \mathrm{O}, l\right)-\left[2 \bar{H}\left(\mathrm{H}_2, \mathrm{~g}\right)+\bar{H}\left(\mathrm{O}_2, \mathrm{~g}\right)\right]\)
Where \(\bar{H}\left(\mathrm{H}_2 \mathrm{O}, l\right), \bar{H}\left(\mathrm{H}_2, \mathrm{~g}\right) \text { and } \bar{H}\left(\mathrm{O}_2, \mathrm{~g}\right)\) are the molar enthalpies of H2O(g) , H2(g) and O2(g) , respectively.
Relation between heat of reaction at constant volume [qv) and heat of reaction at constant pressure (qp)
If a reaction is carried out at a fixed pressure than the heat of the reaction, qp = change in enthalpy in the reaction, ΔH. …………………(1)
If the reaction is carried out at constant volume then the heat of the reaction, qv = change in internal energy in the reaction, ΔU …………………(2)
If the changes in internal energy and volume in a reaction occurring at constant pressure are AUp and AV, respectively, then according to the relation H = U + PV
⇒ \(\Delta H=\Delta U_P+\Delta(P V)=\Delta U_P+P \Delta V\)
[As pressure (P) is constant, Δ(PV) = PΔV
∴ \(q_P=\Delta H=\Delta U_P+P \Delta V\) …………………(3)
since qp = ΔH
Subtracting equation [2] from [3], we obtain
∴ \(q_P-q_V=\Delta U_P+P \Delta V-\Delta U=\left(\Delta U_P-\Delta U\right)+P \Delta V\)
At a particular temperature, the difference between AUp (change in internal energy at constant pressure) and AU (change in internal energy at constant volume) is very small. Therefore, it is considered that
⇒ \(\Delta U_P \approx \Delta U.\)
Since qp -qv = PΔV
Or, ΔH-ΔU = PΔV ………………………….(4)
In the case of solids and liquids:
For reactions involving only solids and liquids, the change in volume (ΔV) of the reaction system is negligibly small. So, in such reactions, the heat of the reaction at constant pressure (qp or ΔH) becomes equal to the heat of the reaction at constant volume (qV or ΔU).
In the case of gases:
For a reaction involving gaseous substances
Example:
⇒ \(A(g)+B(g) \rightarrow C(g) \text { or } A(g)+B(g) \rightarrow C(I)\),
Or \(A(s) \rightarrow B(s)+C(g)\), etc.]
The change in volume (Δ V) of the reaction system may be sufficiently high. We can determine the value of PΔV as illustrated below
Let us consider a gaseous reaction that occurs at constant pressure (P) and temperature (T).
Suppose, n1 and V1 are the total number of moles and the total volume of the reactant gases, respectively, and n2 and IA, are the Here, total number of moles and the total volume of the product gases respectively. If the gases are assumed to behave ideally, then for gaseous reactants PV1 = n1RT., and gaseous products PV2 = n2RT.
∴ \(P\left(V_2-V_1\right)=\left(n_2-n_1\right) R T \text { or, } P \Delta V=\Delta n R T\)
Substituting ΔnRT for PΔV into equation (4) we obtain
⇒ \(q_p-q_V=\Delta n R T \text { and } \Delta H-\Delta U=\Delta n R T\)
∴ \(\left[\boldsymbol{q}_p=\boldsymbol{q}_V+\Delta n \| T\right] \text { and }[\Delta I=\Delta U+\Delta n \ RT] \cdot \cdot \cdot \cdot \cdot [5]\)
Using equation (5), ΔH (or qp) can be calculated from the known value of U (or Δv), and ΔU (or qV) can be calculated from the known value of AII (or ΔyU). If
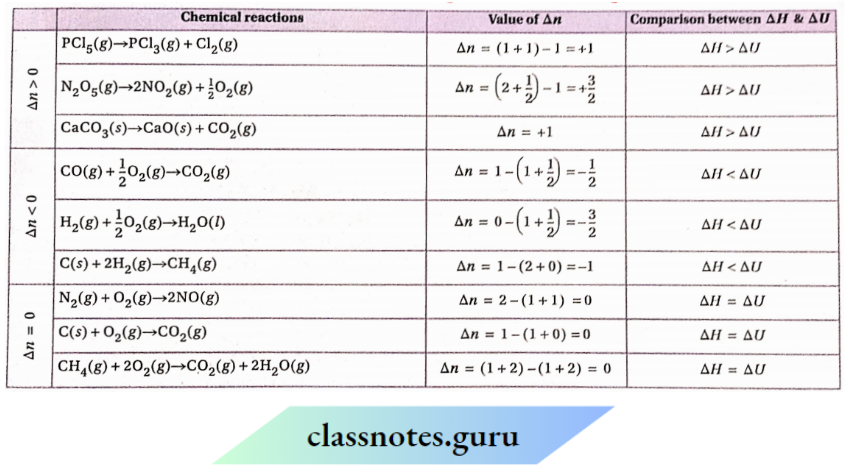
Δn=0; ΔH=ΔU;Δn>0,ΔH>ΔU;Δn>0,ΔH<ΔU.
Comparison between the values of ΔH and ΔU for gaseous reaction having different Δn values:
Standard State And Standard Reaction Enthalpy
Enthalpy change in a reaction depends on the conditions of temperature, pressure, and physical states of the reactants and products. To compare enthalpies of different reactions, we define a set of conditions called standard state, at which the values of AH for different reactions are calculated.
Standard State:
The standard state ofa substance is defined as the most stable and purest state of that substance at the temperature of interest and atm pressure. In the definition of the standard state, temperature is not specified like pressure (1 atm). If the temperature is not mentioned, then 25 °C (298.15 K) is taken as a reference temperature. However, this does not mean 25 °C is the standard temperature. A pure substance can have different standard states depending on the temperature of interest, but in each of these states, the pressure is always 1 atm.
Examples:
- The standard state of liquid water at a particular temperature means H2O(l) at that temperature and 1 atm pressure.
- The standard state of ice at a particular temperature means pure H2O(s) at that temperature and atm pressure.
- The standard state of liquid ethanol at 25 °C means C2H5OH(l) at 25 °C and 1 atm pressure.
Standard enthalpy of reaction:
The standard enthalpy change of a reaction is defined as the enthalpy change that occurs when the stoichiometric number of moles of reactants, indicated by the balanced chemical equation of the reaction, is completely converted into products at a particular temperature and l atm (i.e., at the standard state).
The standard enthalpy of reaction at a particular temperature ( T K) is denoted by \(\Delta H_T^0\). The superscript ‘0’ indicates the standard state, and the subscript T indicates the temperature in the Kelvin scale.
Explanation:
⇒ \(\mathrm{C}_3 \mathrm{H}_8(\mathrm{~g})+5 \mathrm{O}_2(\mathrm{~g}) \rightarrow 3 \mathrm{CO}_2(\mathrm{~g})+4 \mathrm{H}_2 \mathrm{O}(l)\)
For this reaction \(\Delta H_{2 \mathrm{qg}}^0=-2220 \mathrm{~kJ}\) indicating that at.
- 298 K temperature and atm pressure, if lmol of propane (C3H8) and mol of O2 react completely to form 3 mol of CO, and 4 mol of water at the same temperature and pressure (i.e, 29S K and atm respectively), then 2220 kj of heat will be evolved.
- Alternatively, it can be said that at 298K temperature and 1 atm pressure, the change in enthalpy for the following reaction is =-2220 kJ.

Factors affecting the reaction enthalpy
1. Physical states of reactants and products:
During the change of physical states of a substance (like solid, solid→ liquid, liquid→ vapor, etc.) heat is either absorbed or evolved. Thus, the value of the heat of the reaction or enthalpy of the reaction depends upon the physical states ofthe reactants and products. For example, in the following two reactions, due to the different physical states ofthe products,
The values ofthe heat of the reaction are different:
⇒ \(2 \mathrm{H}_2(g)+\mathrm{O}_2(g) \rightarrow 2 \mathrm{H}_2 \mathrm{O}(l) ; \Delta H=-571.6 \mathrm{~kJ}\)
⇒ \(2 \mathrm{H}_2(g)+\mathrm{O}_2(g) \rightarrow 2 \mathrm{H}_2 \mathrm{O}(g) ; \Delta H=-483.6 \mathrm{~kJ}\)
2. The condition under which the reaction is conducted:
At a particular temperature, a chemical reaction can be conducted either at constant pressure or at constant volume. If a reaction occurs at constant pressure, then the heat of reaction (qp)
⇒ \(=H_{\text {product }}-H_{\text {reactant }}=\Delta H\)
Occurs at constant volume, then the heat of reaction (qp) — The relation between ΔH and ΔH is ΔH = ΔH + PΔV The quantity PAV indicates pressure-volume work.
- So, the difference between ΔH and AH is equal to the pressure-volume work involved during the reaction.
- If the volume of the reacting system remains fixed (ΔV = 0), then pressure-volume work = 0 and ΔH = ΔH.
- If the volume ofthe reacting system changes (ΔV=0), then, the pressure-volume work, PΔV≠0 and ΔH≠ΔU.
3. Allotropic forms of the reacting elements:
As the different allotropic forms have different enthalpies, the value of reaction enthalpy depends upon the allotropic forms of the reactants.
For example, the enthalpies of the reaction are different for the oxidation of graphite and diamond (two allotropic forms of carbon)
⇒ \(\mathrm{C}(\text { graphitè, } s)+\mathrm{O}_2(\mathrm{~g}) \rightarrow \mathrm{CO}_2(\mathrm{~g})\)
ΔH=-393.5 kJ
⇒\(\mathrm{C}(\text { diamond, } s)+\mathrm{O}_2(g) \rightarrow \mathrm{CO}_2(g)\)
Δ H =-395.4 kJ
4. Amount of the reactants:
As enthalpy is an extensive property, the magnitude of enthalpy change in a reaction (ΔH) is proportional to the amount of reactants undergoing the reaction.
For example, in the reactions given below, the magnitude of ΔH (reaction enthalpy) for reaction (1) is twice that for the reaction (1). This is because the total number of moles of reactants in the reaction (2) is twice as many as that in the reaction (1).
⇒ \(\mathrm{H}_2(g)+\frac{1}{2} \mathrm{O}_2(g) \rightarrow \mathrm{H}_2 \mathrm{O}(l), \Delta H=-285.8 \mathrm{~kJ}\) ……………………………..(1)
⇒ \(2 \mathrm{H}_2(g)+\mathrm{O}_2(g) \rightarrow 2 \mathrm{H}_2 \mathrm{O}(l), \Delta H=-571.6 \mathrm{~kJ}\) ……………………………..(2)
5. Temperature:
Temperature has a significant effect on the reaction enthalpy of a reaction. The extent of temperature dependence of the reaction enthalpy depends on the nature of the reaction.
Heat Of Reaction Numerical Examples
Question 1. The value of ΔH for the given reaction at 298K is — 282.85 kj. mol-1. Calculate the change in internal energy: \(\mathrm{CO}(g)+\frac{1}{2} \mathrm{O}_2(\mathrm{~g}) \rightarrow \mathrm{CO}_2(\mathrm{~g})\)
Answer:
We know, ΔH = ΔH + ΔnRT where, Δn = (total number of moles of the gaseous products) — (total number of moles of the gaseous reactants)
For the given reaction, ,\(\Delta n=1-\left(1+\frac{1}{2}\right)=-\frac{1}{2}\)
As per given data, ΔH = -282.85 kj-mol-1 & T = 298 K
∴ \(-282.85=\Delta U+\left[\left(-\frac{1}{2}\right) \times 8.314 \times 10^{-3} \times 298\right]\)
∴ \(\Delta U=-281.61 \mathrm{~kJ} \cdot \mathrm{mol}^{-1}\)
Question 2. The bond energy of a diatomic molecule IN given as the change in internal energy due to dissociation of that molecule. Calculate the bond energy of O2. Given: \(\mathrm{O}_2(\mathrm{~g}) \rightarrow 2 \mathrm{O}(\mathrm{g}) ; \Delta H=498.3 \mathrm{~kJ} \cdot \mathrm{mol}^{-1}, T=298 \mathrm{~K}\)
Answer:
In the dissociation reaction of O2 molecules, Δn = 2-1 = 1.
We know, ΔH = ΔU + ΔnRT
As given, ΔH = 498.3 kj.mol-1 , T = 298 K
∴ \(498.3=\Delta U+\left(1 \times 8.314 \times 10^{-3} \times 298\right)\)
or, ΔU= 495.8J
∴ Bond energy of O2 molecule = 495.8 kj.mol-1
Question 3. Calculate the values of ΔH and ΔH in the vaporization of 90 g of water at 100°C and 1 atm pressure. The latent heat of vaporization of water at the same temperature and pressure = 540 cal g-1.
Answer:
⇒ \(90 \mathrm{~g} \text { of water }=\frac{90}{18}=5 \mathrm{~mol} \text { of water. }\)
Vaporisation of water:
⇒ \(\mathrm{H}_2 \mathrm{O}(l) \rightarrow \mathrm{H}_2 \mathrm{O}(\mathrm{g})\)
Therefore, in the vaporization of1 mol of water, Δn = +1.
Hence, for the vaporization of 5 mol water, Δn = +5.
So, the amount of heat required to vaporize 90 g (5 mol) of water =540 x 90 = 48600 cal.
As the vaporization process occurs at constant pressure (1 atm), the heat absorbed = enthalpy change.
∴ The change in enthalpy in the vaporization of 90g of water, ΔH = 48600 cal
∴ The change in internal energy in the vaporization of 90gofwater, ΔH = ΔH-ΔnRT
= 48600- 5 × 1.987 × (273 + 100) = 44894.24 cal .
Question 4. Assuming the reactant and product gases obey its ideal gas law, calculate the change in internal energy (AE) at 27°C for the given reaction:
Answer:
⇒ \(\mathrm{C}_2 \mathrm{H}_4(\mathrm{~g})+3 \mathrm{O}_2(\mathrm{~g}) \rightarrow 2 \mathrm{CO}_2(\mathrm{~g})+2 \mathrm{H}_2 \mathrm{O}(l) ;\)
In tlui rimdlon, Δn= 2-1=1.
We know, ΔH = ΔU+ΔuRT
As given, ΔH = 498.3 kj. mol-1 , T = 298 K
∴ 498.3 = ΔH + (1 × 8.314 × 10-3 × 298) or, ΔH = 495.8 kJ
The bond energy of the O2 molecule = 495.8 kj.mol-1
In the reaction , Δn =2- (1 + 3)= -2
we know ΔH+ ΔnRT
Or, 337= ΔU-2 × 1.987 × 10-3 × 300
Or, ΔU =-335.8kcal