CBSE Class 12 Physics Chapter 11 Dual Nature Of Radiation And Matter Multiple Choice Questions And Answers

Question 1. The speed acquired by a free electron when accelerated from rest through a potential difference of 100V is:
- 6 x 106 m s-1
- 3 x 106 m s-1
- 4 x 105 m s-1
- 2 x 103 m s-1
Answer: 1. 6 x 106 m s-1
Read and Learn More Important Questions for Class 12 Physics with Answers
Question 2. Photons of energy 1 eV and 2.5 eV successively illuminate a metal whose work function is ________.
- 1:2
- 2:1
- 3:1
- 1:3
Answer: 1. 1:2
K.Emax for 1 photon = 1-0.5 = 0.5eV
K.Emax for 2 photon = 2.5-0.5 = 2eV
⇒ \(\frac{K E_1}{K E_2}=\frac{0.5}{2}=\frac{1}{4} \quad\left(K=\frac{1}{2} m v^2\right)\)
So speed \(\left(\frac{v_1}{v_2}=\frac{1}{2}\right)\)
Question 3. The de-Broglie wavelength associated with a particle with rest mass m0 and moving with the speed of light in vacuum is ________.
- \(\frac{\mathrm{h}}{\mathrm{m}_0 \mathrm{c}}\)
- 0
- ∞
- \(\frac{\mathrm{m}_0 \mathrm{c}}{\mathrm{h}}\)
Answer: 2. 0
Question 4. Photoelectric effect represents __________
- Electron has a wave nature
- Light has a particle nature
- Light has a wave nature
- None of these
Answer: 2. Light has a particle nature
Question 5. If the momentum of an electron is required to be the same as that of a wave having 5200 Å wavelength, its velocity should be ______ ms-1
- 103
- 1.4 x 103
- 1.39 x 103
- 2.8 x 103
Answer: 3. 1.39 x 103
⇒ \(\lambda=\frac{h}{m v}\)
∴ \(v=\frac{h}{m \lambda}=\frac{6.62 \times 10^{-34}}{9.1 \times 10^{-31} \times 5200 \times 10^{-10}}=1.39 \times 10^3 \mathrm{~m} / \mathrm{s}\)
Question 6. To increase the de Broglie wavelength of an electron from 0.5 x 10-10 m to 10-10 m, its energy should be _________.
- Increased to four times
- Halved
- Doubled
- Decreased to the fourth part
Answer: 4. Decreased to fourth part
⇒ \(\lambda=\frac{\mathrm{h}}{\sqrt{2 \mathrm{mK}}}\) ⇒ \(\lambda \propto \frac{1}{\sqrt{\mathrm{K}}}\)
∴ \(K \propto \frac{1}{\lambda^2}\)
λ → twice, K must be \(\left(\frac{1}{4}\right)^{th}\)
Question 7. The energy of the photon is E = hf and its momentum is \(P=\frac{h}{\lambda}\), where λ, is the wavelength of the photon with this assumption speed of the light wave is
- \(\frac{P}{E}\)
- \(\frac{E}{P}\)
- EP
- \(\left(\frac{E}{P}\right)^2\)
Answer: 2. \(\frac{E}{P}\)
⇒ \(E=h f=\frac{h c}{\lambda}\)
⇒ \(c=\frac{E \lambda}{h} \quad\left(\frac{\lambda}{h}=\frac{1}{P}\right)\)
∴ \(c=\frac{E}{P}\)
Question 8. Which of the following physical quantities has the dimension of Planck constant (h)?
- Angular momentum
- Force
- Energy
- Power
Answer: 1. Angular momentum
Question 9. If the photoelectric effect is not seen with the ultraviolet radiations in a given metal, photoelectrons may be emitted with the _________.
- Radio waves
- Infrared waves
- X-rays
- Visible light
Answer: 3. X-rays
Question 10. The work function of _______ is the lowest.
- Platinum
- Caesium
- nickel
- Copper
Answer: 2. Caesium
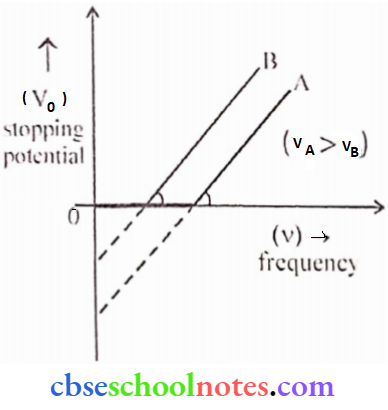
Question 11. The value of slopping potential depends on ________ of incident light.
- Intensity
- Frequency
- Momentum
- Velocity
Answer: 2. Frequency
Question 12. Monochromatic light of frequency 6 x 1014 Hz is produced by laser. Each photon has an energy = ________ J.
- 6 x 1014
- 4 x 10-19
- 4 x 10-20
- 6 x 10-14
Answer: 2. 4 x 10-19
E = hv
E = 6.62 x 10-34 x 6 x 1014 = 39.72 x 10-20 = 3.972 x 10-19 = 4 x 10-19 J
Question 13. For a given frequency of incident radiation, slopping potential ________.
- Does not depend on the intensity
- Is inversely proportional to the intensity
- Is directly proportional to the intensity
- Is inversely proportional to the square of intensity.
Answer: 1. Does do not depend on the intensity
Question 14. The slope of a graph of stopping potential versus frequency of incident radiation is _________.
- c
- h
- \(\frac{h}{e}\)
- \(\frac{e}{h}\)
(where h = Planck’s constant and e = charge of an electron)
Answer: 3. \(\frac{h}{e}\)
Question 15. De-Broglie wavelength of a bullet of mass 0.040 kg travelling at the speed of 1 km/s is _________ m.
- 1.7 x 10-35
- 4.04 x 10-24
- 1. 1 x 10-32
- 3 x 10-32
Answer: 1. 1.7 x 10-35
∴ \(\lambda=\frac{h}{m v}=\frac{6.62 \times 10^{34}}{0.040 \times 1000}=1.655 \times 10^{-35}=1.7 \times 10^{-35} \mathrm{~m}\)
CBSE Class 12 Physics Chapter 11 Dual Nature Of Radiation And Matter Assertion And Reason
For question numbers 1 to 6 two statements are given-one labelled Assertion (A) and the other labelled Reason (R). Select the correct answer to these questions from the codes (1), and (2). (3) and (4) as given below.
- Both A and R are true and R is the correct explanation of A
- Both A and R are true but R is NOT the correct explanation of A
- A is true but R is false
- A is false and R is also false
Question 1. Assertion: The photoelectric effect demonstrates the wave nature of light.
Reason: The number of photoelectrons is proportional to the frequency of light
Answer: 1. Both A and R are true and R is the correct explanation of A
Question 2. Assertion: At saturation, the photoelectric current is maximum.
Reason: At saturation, all the electrons emitted from the cathode can reach the anode.
Answer: 1. Both A and R are true and R is the correct explanation of A
Question 3. Assertion: A photon is not a material particle. It is a quantum of energy.
Reason: The photoelectric effect demonstrates the wave nature of radiation.
Answer: 3. A is true but R is false
Question 4. Assertion: Photo electric current increases if the distance between the cathode and anode is increased.
Reason: The momentum of a photon is directly proportional to its wavelength.
Answer: 4. A is false and R is also false
Question 5. Assertion: The photosensitivity of a metal is high if its work function is small.
Reason: Work function = hv0, where v0 is the threshold frequency.
Answer: 2. Both A and R are true but R is NOT the correct explanation of A
Question 6. Assertion: In his study of photoelectric emission, Hallwachs connected a negatively charged zinc plate to an electroscope. He found that negatively charged particles were emitted from the zinc plate under the action of visible light.
Reason: An uncharged zinc plate becomes positively charged when it is irradiated by visible light.
Answer: 4. A is false and R is also false
CBSE Class 12 Physics Chapter 11 Dual Nature Of Radiation And Matter Short Questions And Answers
Question 1. A proton and a particle are accelerated through the same potential difference. Which one of the two has
- Greater de-Broglie wavelength, and
- Less kinetic energy? Justify your answer
Answer:
∴ \(\lambda=\frac{h}{p}=\frac{h}{\sqrt{2 q V m}}\)
1. α – particle: \(\frac{h}{\sqrt{2 q_{\alpha} V_{m_{\alpha}}}}=\lambda_\alpha\) ( ∵ \(\begin{aligned} & \mathrm{q}_\alpha=2 \mathrm{q}_{\mathrm{p}} & \mathrm{m}_{\mathrm{\alpha}}=4 \mathrm{~m}_{\mathrm{p}} \end{aligned}\))
proton: \(\frac{h}{\sqrt{2 q_p V m_p}}=\lambda_p\)
Clearly, \(\lambda_{\mathrm{p}}>\lambda_\alpha \text { as } \mathrm{m}_\alpha>\mathrm{m}_{\mathrm{p}} \ and \mathrm{q}_\alpha>\mathrm{q}_{\mathrm{p}}\)
So, a proton has a greater de-broglie wavelength.
2. As \(\frac{1}{2} m v^2=q_V ;(\text { K.E. })_{\mathrm{p}}<(\text { K.E. })_{\alpha} \text { as } \mathrm{q}_{\mathrm{p}}<\mathrm{q}_{\alpha}\)
So, a proton has less K.E
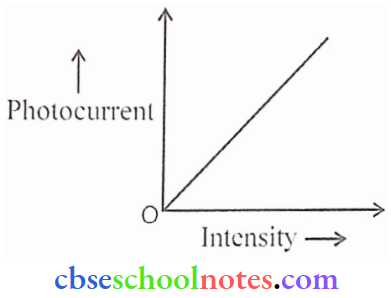
Question 2.
- Draw graphs showing the variation of photoelectric current with applied voltage for two incident radiations of equal frequency and different intensities. Mark the graph for the radiation of higher intensity.
- Name the phenomenon which shows the quantum nature of electromagnetic radiation.
Answer:
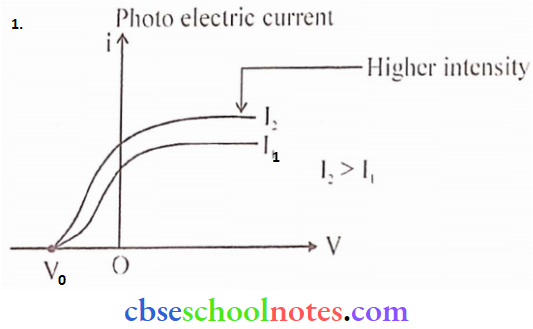
The graph I2 corresponds to radiation of higher intensity
2. Photoelectric effect (PEE)
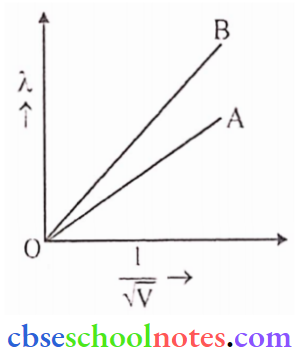
Question 3. Plot a graph showing the variation of de-Broglie versus \(\frac{1}{\sqrt{V}}\) where V is accelerating the potential for two particles A and B carrying the same charge but of masses m1, m2, (m1 > m2). Which one of the two represents a particle of smaller mass and why?
Answer:
As, \({\lambda} =\frac{h}{\sqrt{2 \mathrm{mqV}}}\)
Graph: Slope of verses \(\frac{1}{\sqrt{V}}\) graph will be inversely prop, to the square root of the mass of the particles. Now, the slope of B is greater and it shows that its mass is smaller.
Question 4. The wavelength λ of a photon and the de-Broglie wavelength of an electron have the same value. Show that the energy of a photon is (2λmc/h) limes the kinetic energy of electron: where m. c and h have their usual meaning.
Answer:
⇒ \(E_{\text {photon }}=\frac{h c}{\lambda}\) → (1) \(\lambda_{\text {photon }}=\lambda_{\text {electron }}={\lambda}\)
⇒ \(E_{\text {elcetron }}=\frac{h^2}{2 m \lambda^2}\) → (2) ( ∵ \(\lambda=\frac{\mathrm{h}}{\sqrt{2 \mathrm{mE}_{\text {electron }}}}\)))
Dividind (1) by (2), \(\frac{\mathrm{E}_{\text {photon }}}{\mathrm{E}_{\text {electron }}}=\frac{2 \mathrm{mc \lambda}}{\mathrm{h}}\)
Question 5. Calculate the dc-Broglic wavelength of the electron orbiting in the n = 2 stale of hydrogen atom.
Answer:
The velocity of an e– in the first orbit (n = 1) of the hydrogen atom, v = 2.18 x 106 m/s.
Now, the velocity of e– in the second orbit (n = 2) of the hydrogen atom will be given as, v’ = v/n
∴ v’ = 1.09 x 106 m/s
So mv’ = 9.9 x 10-25
Putting the values in \(\lambda=\mathrm{h} / \mathrm{mv}^{\prime}=\frac{6.6 \times 10^{-34}}{9.9 \times 10^{-25}}=6.68 Å\)
Question 6.
- Define the intensity of radiation based on a photon picture of light. Write its S.I. unit.
- Draw a plot showing the variation of the de-Broglie wavelength of an electron as a function of its K.E.
Answer:
The intensity of radiation is defined as the energy associated with several photons incident/could from a unit surface area in unit time.
i.e. \(\text { Intensity }=\frac{\text { Energy }}{\text { Area } \times \operatorname{time}}\)
SI unit:
∴ \(\frac{\text { joule }}{m^2-s} \text { or } \mathrm{W}-\mathrm{m}^{-2}\)
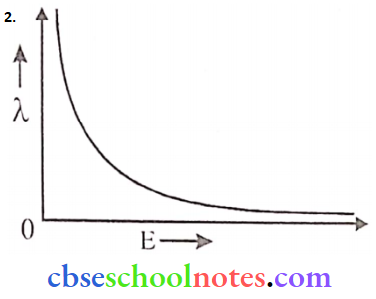
∵ \(\lambda=\frac{\mathrm{h}}{\sqrt{2 \mathrm{mE}}}\)
Question 7.
- Name the factor on which photoelectric emission from a surface depends.
- Define the term ’threshold frequency’ for a photosensitive material.
Answer:
- Metal should be photo-sensitive.
- For a given photosensitive material, threshold frequency is the minimum frequency of radiation that is required for photoelectric emission from the material.
Question 8.
- Plot a graph showing the variation of photocurrent v/s collector potential for three different intensities I1 < I2 < I3, two of which (I1 and I2) have the same frequency v and the third has frequency v1 > v2.
- Explain the nature of the curves based on Einstein’s equation.
Answer:
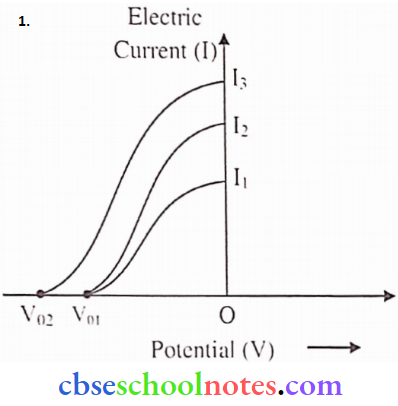
2. From. eV0 = hv- hv0 [Clearly stopping potential (V0) depends on incident freq.(v)]
or \(V_0=\frac{h}{e} v-\frac{h}{e} v_0\)
So. for I1 and I2 intensities, freq. (v) is the same, hence for them stopping potential (V0) is also equal.
Further, for. intensity having freq (v1) stopping potential is more. (∵ V1 > V2 )
Question 9. For the light of wavelength 400 nm incident on the cathode of a photocell, the stopping potential is 6V. If the wavelength of incident light is increased to 600 nm. calculate the new stopping potential. (Take h =4.14 x 10-15 eV. s)
Answer:
Given: \(\mathrm{hc}=4.14 \times 10^{-15} \times 3 \times 10^8 \mathrm{eV}s . \frac{\mathrm{m}}{\mathrm{s}}=12.42 \times 10^{-7} \mathrm{eVm}=12420 \mathrm{eVA}\)
λ1= 400 nm, λ2= 600 nm
V01 =6V, V02 = ?
∵ E = W0 + K.Emax
K.Emax = E – W0
⇒ \(\mathrm{eV}_0=\frac{h \mathrm{c}}{\lambda}-\mathrm{W}_0\) → (1)
So, \(\mathrm{eV}_{01}=\frac{h c}{\lambda_{1}}-W_0\) → (2)
⇒ \(\mathrm{eV}_{02}=\frac{\mathrm{hc}}{\lambda_2}-\mathrm{W}_0\) → (3)
Equation (3) – (2)
⇒ \(\left(\mathrm{V}_{02}-\mathrm{V}_{01}\right) \mathrm{e}=\mathrm{hc}\left(\frac{\mathrm{I}}{\lambda_2}-\frac{1}{\lambda_1}\right) \Rightarrow\left(\mathrm{V}_{02}-6\right)=\frac{\mathrm{hc}}{\mathrm{e}}\left(\frac{1}{600}-\frac{1}{400}\right)\)
∴ \(V_{02}-6=\frac{12420Å \mathrm{eV}}{\mathrm{e}}\left(-\frac{1}{12000}\right) \frac{1}Å; \quad V_{02}=\frac{-12420}{12000}+6=4.965 \mathrm{~V} \simeq 5 \mathrm{~V}\)
Question 10. Write three characteristic features in the photoelectric effect which cannot be explained based on the wave theory of light, but can be explained only using Einstein’s equation.
Answer:
- The instantaneous ejection of photoelectrons.
- Existence of threshold freq. for a metal surface.
- The fact that the K.E. of the emitted electrons is independent of the intensity of the light depends on its frequency.
Question 11. Plot a graph showing the variation of photoelectric current with intensity of light. The work function for the following metals is given:
Na: 2.75 eV and Mo: 4.17 eV
Which of these will not give photoelectron emission from a radiation of wavelength 3300 Å from a laser beam? What happens if the source of the laser beam is brought closer?
Answer:
For, \(\lambda=3300Å . E=\frac{h c}{\lambda}=\frac{6.6 \times 10^{-34} \times 3 \times 10^8}{3300 \times 10^{-10}}=6 \times 10^{-19} \mathrm{~J}=\frac{6 \times 10^{-19}}{1.6 \times 10^{-19}} \mathrm{eV}=3.75 \mathrm{eV}\)
Incident energy 3.75 eV <4.17 eV, so the photoelectric effect will not take place in Mo (4. 17 eV). Photocurrent will increase in Na if the source of the laser beam is brought closer.
Question 11. State two important properties of photons which are used to write Einstein’s photoelectric equation. Define
- Stopping potential and
- Threshold frequency, using Einstein’s equation and drawing necessary plots between relevant quantities.
Answer:
- The energy of a photon is proportional to the freq. of light. (E = hv)
- Photons are quanta or discrete carriers of energy.
Stopping potential: In the experiment of the photoelectric effect, the value of the negative potential of the anode at which photoelectric current reduces to zero is called slopping potential for the given freq. of incident radiation.
Threshold freq.: For a given material, there exists a certain min. frequency below which no photoelectron can come out from the metal surface. This is called threshold frequency.
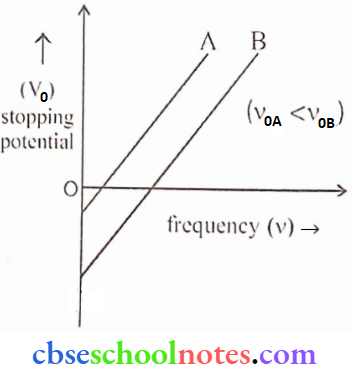
Question 12. Sketch the graphs showing the variation of stopping potential with the frequency of incident radiations for two photosensitive materials A and B having threshold frequencies vA > vB.
- In which case is the slopping potential more and why?
- Does the slope of the graph depend on the nature of the material used? Explain.
Answer:
From eV0 = Kmax = hv – Φ
eV0 = hv- hv0
∴ \(V_0=\frac{h v}{e}-\frac{h v_0}{e}\)
1. From the above equation, we can conclude, that the more threshold freq., the less would be the slopping potential (V0). Here, v0A > v0B, hence slopping potential (V0) is greater for B than A.
2. \(V_0=\left(\frac{h}{c}\right) v-\frac{h}{c} v_0(y=m x \pm c)\)
The slope of the above equation is \(\left(\frac{\mathrm{h}}{\mathrm{c}}\right)\) which is ‘independent’ of the nature of the material.
Question 13. Using a photon picture of light, show how Einstein’s photoelectric equation can be established. Write two features of the photoelectric effect which cannot be explained by wave theory.
Answer:
Einstein states that electromagnetic radiation energy is built up of discrete units called quanta of energy and has energy hv where ‘h’ is Planck’s constant and ‘V’ is the frequency of light.
In this phenomenon, an e– absorbs a quantum of energy (hv), if the energy absorbed exceeds the minimum energy needed to escape from metal (Φ0 ), e– is emitted with maximum K.E.
∴ \(\mathrm{K}_{\text {max }}=h v-\phi_0\) [Einstein’s photo electric equation]
Two features of PEE which can’t be explained by wave theory
- A min. frequency (threshold frequency) exists for diff. metals below which PEE is not possible.
- PEE is an instantaneous phenomenon.
Question 14. Define the term ‘cut-off frequency” in photoelectric emission. The threshold frequency of a metal is f. When the light of frequency 2f is incident on the metal plate, the maximum velocity of photo-electrons is V1. When the frequency of the incident radiation is increased to 5f, the maximum velocity of photo-electrons is v2. Find the ratio: v1: v2.
Answer:
Cut-off Frequency: The minimum. frcq. of incident light which can emit photoelectrons from a material is known as cut-off frequency.
From eq. KEmax = hv- hv0
⇒ \(\frac{1}{2} m v_1^2=h(2f)-hf=hf\) → (1)
⇒ \(\frac{1}{2} m v_2^2=h(5f)-hf=4 hf\) → (2)
∴ \(\frac{v_1^2}{v_2^2}=\frac{1}{4} \Rightarrow \frac{v_1}{v_2}=\frac{1}{2} \Rightarrow v_1: v_2=1: 2\)
Question 15. The given graph shows the variation of photo-electric current (I) with the applied voltage (V) for two different materials and for two different intensities of the incident radiation. Identify and explain using, Einstein’s photoelectric equation the pair of curves that correspond to
- Different materials but the same intensity of incident radiation,
- Different intensities but the same materials.
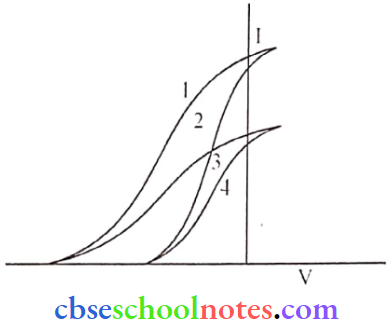
Answer:
- (1,2) and (3,4) are diff. materials of the same intensity. The saturation current is the same but the stopping potential is different.
- ( 1,3) and (2,4) have diff. intensities but the same materials. As saturation currents arc differently slopping potential is the same.
Question 16.
- Calculate the frequency of a photon of energy 6.5 x 10-19 J.
- Can this photon cause the emission of an electron from the surface of Cs of work function 2.14 eV? If yes, what will be the maximum kinetic energy of the photoelectron?
Answer:
1. Energy E=hv
∴ \(v=\frac{E}{h}=\frac{6.5 \times 10^{-19}}{6.63 \times 10^{-34}}=0.98 \times 10^{15}=9.8 \times 10^{14} \mathrm{Hz}\)
2. Work function Φ0 = 2.14 eV
The energy of photons in eV
∴ \(E=\frac{6.5 \times 10^{-19}}{1.6 \times 10^{19}}=4.06 \mathrm{eV}\)
KEmax = E – Φ0 = 4.06 – 2.1 4 = 1 .92 eV
CBSE Class 12 Physics Chapter 11 Dual Nature Of Radiation And Matter Long Questions And Answers
Question 1. The photoelectric emission is possible only if the incident light is in the form of packets of energy, each having a definite value, more than the work function of the metal. This shows that light is not of wave nature but of particle nature. It is for this reason that photoelectric emission was accounted for by the quantum theory of light.
(1). Packets of energy are called
- Electron
- Quanta
- Frequency
- Neutron
Answer: 2. Quanta
(2). Energy associated with each photon
- hc
- mc
- hv
- hk
Answer: 3. hv
(3). Which of the following waves can produce the photoelectric effect
- UV radiation
- Infrared radiation
- Radio waves
- Microwaves
Answer: 1. UV radiation
(4). The work function of alkali metals is
- Less than zero
- Just equal to other metals
- Greater than other metals
- Quite less than other metals
Answer: 4. Quite less than other metals
Question 2. According to de-Broglie a moving material particle sometimes acts as a wave and sometimes as a particle or a wave is associated with a moving material particle which controls the particle in every respect. The wave associated with moving material particles is called matter wave or de-Broglie wave whose wavelength called dc-Broglic wavelength, is given by λ = h/mv.
(1). The dual nature of light is exhibited by
- Diffraction and photoelectric effect
- Photoelectric effect
- Refraction and interference
- Diffraction and reflection.
Answer: 1. Diffraction and photoelectric effect
(2). If the momentum of a particle is doubled, then its de-Broglie wavelength will:
- Remain unchanged
- Become four times
- Become two times
- Become half
Answer: 4. Become half
(3). If an electron and proton are propagating in the form of waves having the same X, it implies that they have the same:
- Energy
- Momentum
- Velocity
- Angular momentum
Answer: 2. Momentum
(4). Velocity of a body of mass m, having de-Broglie wavelength λ, is given by the relation:
- v = λ h/m
- v = λ m/h
- v = λ/hm
- v = h/λm
Answer: 4. v = h/λm
