Quantum Number
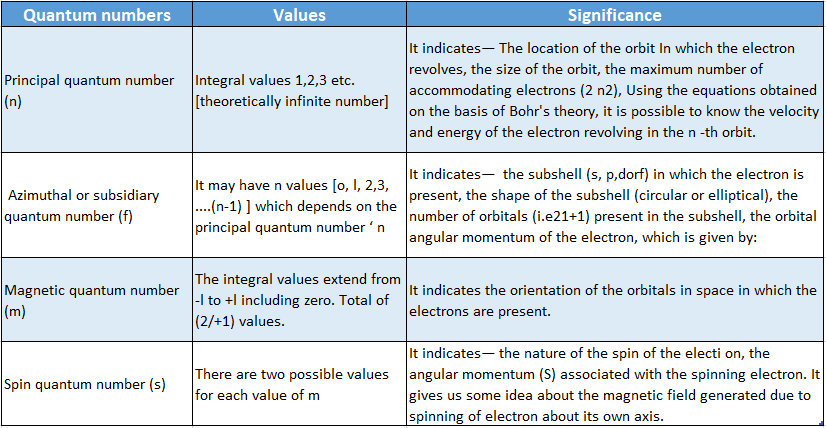
Quantum Number Definition: A set of four numbers that provide complete information about any electron in an atom are known as quantum numbers.

Quantum Number Classification: The four quantum numbers are—
- Principal Quantum Number (N)
- Azimuthal Or Subsidiary quantum number (l)
- Magnetic quantum number (m or m1)
- Spin quantum number (5 or mg ).
- To specify an electron in an atom, the following four quantum numbers should be mentioned.
- Principal quantum number [n]
Quantum Number Origin:
- From Bohr’s postulates, it is known that each electronic orbit surrounding the nucleus in an atom represents an energy level.
- The average energy of the electrons revolving in a particular orbit is fixed. So, these orbits are called principal energy levels or principal quantum levels.
- Depending on their distance from the nucleus, these orbits or principal energy levels are designated by the numbers 1,2,3, 4… etc. These numbers 1,2,3,4… etc. are called principal quantum numbers.
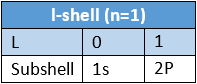
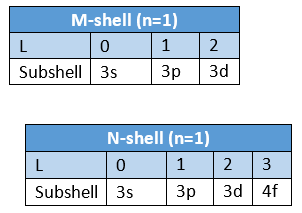
Quantum Number Designation: The principal quantum number is denoted by the letter ‘n ’. For AT-shell n = 1, for L -shell n = 2, for Mshell n = 3 and so on.
Read and Learn More CBSE Class 11 Chemistry Notes
Information obtained:
- The higher the value of n, the greater the distance of the orbit from the nucleus, and hence, the greater the size ofthe orbit. Thus, r1<r2<r3< r4< …
- The higher the value of ‘ n,’ the greater will be the electronic energy associated with the orbit.
- Thus, El<E2<E3<E4<………..
- A maximum number of electrons that can be accommodated in a principal quantum level n is given by the formula 2n2.
- Limitations of 2β2 The maximum number of electrons in any orbit can never be more than 32 even if the value of n exceeds 4.
- The outermost electronic shell does not contain more than 8 electrons.
- The penultimate shell (i,e., the shell just preceding the outermost shell) does not contain more than 18 electrons.
Azimuthal or subsidiary quantum number
Azimuthal or subsidiary quantum number Origin: A spectrograph with high resolving [/]power has revealed that each bright line in the spectrum of atomic hydrogen consists of some closely spaced finer lines.
This fact suggests that each orbit or energy level in an atom is composed of subshells. Electrons occupying these subshells within the same -shell, exhibit slight differences in energy.
In order to explain the formation of the fine structure of spectral lines, Sommerfeld proposed
the existence of elliptical orbits, besides Bohr’s circular
orbits.
To specify the shape of the elliptical orbit, another supplementary quantum number is necessary.
This supplementary quantum number which indicates the captivity of the electronic orbit is called azimuthal or subsidiary quantum number denoted by the letter.
If the principal quantum number of any orbit is n, then the total number of subshells incorporated in that orbit will also be n.
Magnitude:
- As per quantum mechanical calculations, the angular momentum of a moving electron in an elliptical path is given by, L = Jl(l+ 1) X.
- This is often called orbital angular momentum.
- The value of l determines the shape of the path. So, with the help of the principal quantum number and azimuthal quantum number, a precise idea about the size and shape of the electronic path can be obtained.
- If n stands for the principal quantum number of an electronic orbit, the values of l will be from to (n- 1) i.e., with respect to the value of principal quantum number n, the azimuthal quantum number / may assume n number of different values including zero, e.g., for n = 4, 1=0, 1, 2 and 3.
- To indicate the subshells within a shell, spectroscopic symbols are used instead ofthe numbers 0, 1, 2, 3 etc.
- The symbols s, p, d,f, etc., (spectroscopic coinage) are merely the first letters ofthe words sharp, principal, diffuse, and fundamental, used extensively in spectral analysis.
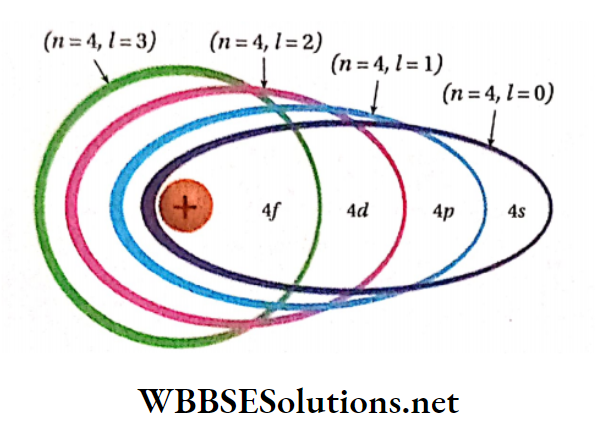
- To express the position of an electron in the atom, the principal quantum number should be written first followed by the symbol of the azimuthal quantum number to its right side, e.g., the subshells included in K, L, M, and N-shells are represented as

- The ratio of the major axis to the minor axis of an elliptical path is given by = (/ + 1)/n .
- An elliptical path for which l = (rc- 1), becomes circular e.g., in the case of 4-orbit if 1 = 3, then that orbit becomes circular. The greater the difference between the values of l and n, the larger the ellipticity of that path.
- The penetrating power and screening effect of an elliptical orbit increases on increasing the ellipticity of the orbit.
- So the penetrating and screening powers of different subshells within the same shell follow the sequence: s> p> d>f.
- Due to the difference in the internal energies of the subshells [s, p, d, f, etc.), the electrons moving in those subshells also possess different energies. Energy associated with the subshells in a particular orbit increases in the following order: s <p<d<f.
Magnetic quantum number (m or mt)
Origin:
- Zeeman in 1896 observed that each fine line in atomic spectra splits further into finer lines in the presence of the highly powerful magnetic field.
- In the absence of a magnetic field, such finer splitting i.e., hyperfine splitting disappears. This phenomenon is called the Zeeman effect. To explain the Zeeman effect, a third type of quantum number, known as a magnetic quantum number was introduced.
Discussion:
- Due to the angular motion of electrons around the nucleus, a magnetic field is produced, which interacts with the external magnetic field.
- As a result subshells of definite energy split into three-dimensional spatial regions called orbitals.
- Magnetic quantum number (MI) signifies the orientation of the orbitals in space in which the electron exists.
- The value of m depends on the azimuthal quantum number l.
- For a certain value of l, m has an o total of (2Z +1 ) different values. These values may be any whole number starting from -Z to +1 (including zero).
- For s- subshell, l = 1 and m – 1. This subshell, l = 0 and m – 0. This orbital (i.e., s-orbital). Z = 1 denotes p -subshell consisting of three orbitals which are directed along three axes.
- These are marked as px, py, and pz orbitals which have the respective values of m = -1, 0, and + 1 . Similarly, d and /-subshells contain 5 and 7 orbitals respectively.
- The negative values of the magnetic quantum number signify that these orbitals are inclined in the direction opposite to the magnetic field and the positive values indicate that these orbitals are inclined in the direction ofthe magnetic field.
- shows the different directions of the d -d-subshell (Z = 2) in the magnetic field.
Orientation of different orbitals of ZV-shell (n = 4] under the influence of magnetic field.
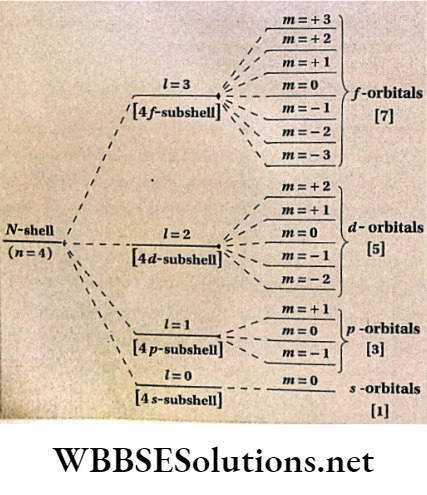
Values of magnetic quantum number (m] for different values of azimuthal quantum number [l]
Spin quantum number [s or ms]
Uhlenbeck and Goudsmit introduced a fourth quantum number called the spin quantum number.
This is because the other three quantum numbers were not able to give sufficient explanation to the hyperfine structure of the atomic spectra.
% Just like the earth, an electron while moving around the nucleus also spins about its own axis either in a clockwise or in an anti-clockwise direction,
Each type of spin can give rise to characteristic spectral lines with the formation of a hyperfine spectrum in the spectral series.
The spin quantum number denoted by the symbol ‘s’ expresses two opposite types of spinning motions of each electron.
The spin quantum number ‘s’ can have only two values, \(+\frac{1}{2} \text { and }-\frac{1}{2}\) The positive and negative signs represent two opposite directions of spinning motion of any spinning motion of any spinning motion of electron are very often represented by two arrows pointing in opposite directions,| and.
Q A spinning electron behaves like a tiny magnet with a definite magnetic moment. The angular mentum associated with the spinning electron is given by the mathematical expression.
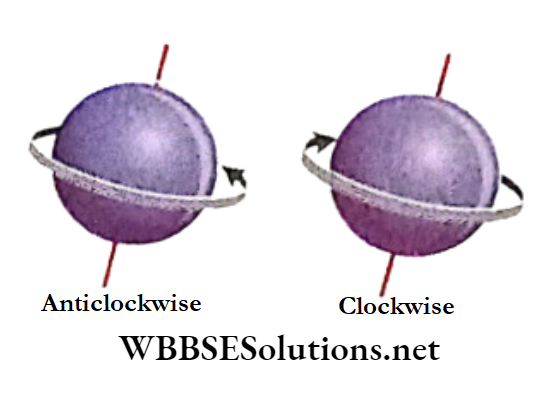
Spin Quantum number (s) signifies the mode of Electron Spin (Clockwise or Anti-clockwise).