Antidotes Introduction
Poison may be defined as any substance that when introduced into or absorbed by a living organism causes illness or death.
Poisoning occurs in many ways
- by accidentally
- by use of recreational substances (such as cannabis, opiates etc.)
- by intentional behaviour
- by occupational exposure
So, as to counteract the effects of a poison, antidotes are used.
Antidotes
An antidote is an agent with specific action against the activity or effect of a poison.
OR
An antidote is an agent which counteracts as a poison.
Whether accidental or intentional, the poisoning requires only sympathomimetic and supportive therapy i.e. removal of poison from the body is first priority in case of poisoning.
It can be done by either gastric lavage or emesis induction (Gastric lavage is the process of cleaning the stomach) while (emesis induction can be done by the administration of substances like activated charcoal to reduce the absorption).
Classification Of Antidotes
The antidotes are classified into three types depending upon their mechanism of action.
Physiological antidotes: They act by producing the effects opposite to that of poison.
For example: Sodium nitrite which converts haemoglobin into methaemoglobin in order to bind cyanide, atropine & physostigmine.
Mechanical antidotes: These render the poison inert by mechanical action or prevent their absorption.
For example: Activated charcoal absorbs the poison to absorption across the intestinal wall, mercuric chloride, sulphanilamide.
Read and Learn More Pharmaceutical Inorganic Chemistry Notes
Chemical antidotes: They change the chemical nature of the poison.
For example:
- Sodium thiosulphate which changes toxic cyanide to non-toxic thiocyanate.
- Sodium sulphate is used to precipitate lead.
- Copper sulphate is used to precipitate phosphorous.
Universal antidote is a combination of physical and cheical antidote. It is an antidote which can be used in cases, where the nature of the poison swallowed is not definitely know. The universal antidote has following composition :
- Powdered animal charcoal
- Tannic acid ‘ • ‘
- Magnesium oxide
Antidotes Reasons Of Poisoning
Poisoning of the body is due to various reasons. It can be due to the presence of heavy metals such as lead, arsenic, mercury, and cadmium; insecticides or pesticides used in our
daily life and cyanide poisoning which occurs due to a variety of occupational sources.
Antidotes Lead Poisoning
Lead poisoning has been recognized as a major public health risk, particularly in developing countries. Though various occupational and public health measures have been under taken in order to control lead exposure.
Lead is regarded as a potent occupational toxin and its toxicological manifestations are well known. In case of severe lead poisoning, Sodium calcium edetate & dimercapol are
commonly used. Chelation therapy has so far been used.
Sodium Calcium Edetate leads from bone and the extra cellular space and then expels it out from the urine. Dimercapol is more effective than the sodium calcium edetate as chelating. It lead from the soft tissues such as brain.
Now a days, Succimer a water soluble analogue of dimercapol has been increasingly preferred. It is suitably administrated by mouth and is well tolerated.
It occurs by a number of ways such as inhalation or ingestion of soluble cyanide salts or cyanide releasing substances like seeds of peach, apricot, cyanamide, cyanogen chloride and
bitter almonds.
It has a characteristic odour of bitter almonds. The symptoms of cyanide poisoning are: Nausea, hypotension, dizziness, drowsiness, coma, convulsions and death.
Cyanide Poisoning
Cyanide poisoning takes place intentionally or accidentally to commit suicide. Two inorganic antidotes are used such as sodium nitrite and sodium thiosulphate to counteract its poisoning.
Nitrate generates ferrous ion of haemoglobin to the ferric ion of methahaemoglobin which has high affinity for cyanide radicals and form cyanomethaemoglobin. However, this
may again dissociate to release cyanide. Therefore, sodium thiosulphate is given to form sodium thiocyanate which is poorly dissociable and is excreted in the urine.

Sodium nitrite is used for this purpose because it has a very weak vasodilator action.
Sodium Nitrite
Chemical formula: NaNO2 Molecular Weight: 69.00
Synonym: Nitrous acid Sodium salt
Preparation
It can be prepared by strongly heating sodium nitrate.

It is more conventinely made by heating the sodium nitrate with metallic lead or carbon which reduces it at lower temperature.
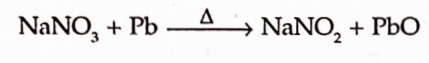
Physical Properties
- It is odourless, colourless to slightly yellow crystals.
- Its taste is saline.
- It is water soluble and sparingly soluble in alcohol.
- It is hygroscopic.
- It is slowly oxidizes to nitrate in air.
Chemical Properties
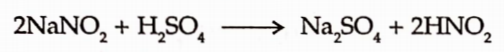
1. Sodium Nitrite is easily decomposed by the acidification with dilute sulphuric acid.
2. It also act as a reducing and oxiding agent.
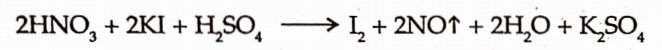
3. With aniline hydrochloride at 4°C, Nitrous acid forms diazonium chloride.

Uses
- It is used in treatment of cyanide poisoning in conjugation with sodium thiosulphate.
- Sodium nitrate is also used as a rust inhibitor preservative in foods such as cured meals for manufacturing dyes.
- It is used as a vasodilator.
Sodium Thiosulphate
Chemical formula: Na2S2O3.5H20 Molecular Weight: 248.2
Synonym: Sodium hyposulphate
Preparation
It can be prepared by boiling sodium sulphite with sulphur.
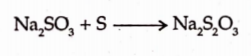
It can be obtained by mixing sulphide liquors sodium carbonate by passing S02 gas.
![]()
It is prepared by passing the sulphur dioxide gas into solution of sodium carbonate.
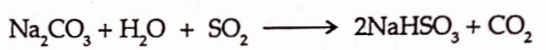
The sodium bisulphite so obtained further reacts with sodium carbonate to give the sulphite.

Physical Properties
- It occurs in the form of large, colourless crystals.
- It is odourless and is having an alkaline taste.
- It is soluble in water but insoluble in alcohol.
- It effloresces in warmy dry air above 33°C.
- When exposed to the moist air, it deliquesces lightly.
Chemical Properties
Sodium thiosulphate when acidified with hydrochloric acid, it decomposes to give sulphur dioxide, water and sulphur.
Thiosulphate act as reducing agent as shown by reaction with the ferric chloride solution.
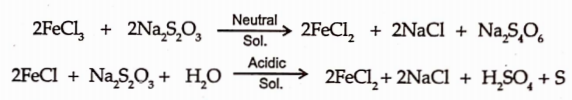
It is also called as antichlor in bleaching due to its reaction with chlorine or hypochlorite

Assay
It is assayed by the iodemetric titration. Dissolve an accurately weighed amount of substance in water & titrate with 0.05M iodine solution using starch mucilage as indicator.
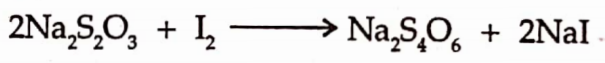
End point is indicated by the presence of blue colour.
Uses
- It is used in the treatment of Cyanide Poisoning. It is also used to treat parasitic skin diseases.
- It is used as antichlor in bleaching process in textile industry.
- It is widely used as stimulant analytical chemical.
Antidotes Short Answer Questions
Question. 1. Differentiate between Poison and Antidote?
Answer. Poison may be defined as any substance that when introduced into or absorbed by living organism causes illness or death while an antidote is an agent which counter
act as a poison.
Question.2. Which kind of therapy is required for Poisoning?
Answer. Poisoning requires sympathomimetic and supportive therapy.
Question.3. How poisoning can be removed immediately from the body?
Answer. Poisoning can be removed immediately from the body by gastric lavage or emesis induction.
Question.4. How poisoning can be occurred?
Answer. Poisoning can occur in many ways:-
- by accidentally
- by use of recreational substances
- by intentional behaviour
- by occupational exposure
Question.5. Classify antidotes.
Answer. Antidotes can be classified into three types
- Physiological antidotes
- Mechanical antidotes
- Chemical antidotes
Question.6. Write down the examples of Mechanical Antidote?
Answer.
- Activated charcoal
- Magnesium sulphate
- Copper sulphate
- Sodium monohydrogen phosphate
Question.7. Which drugs are used in the treatment of lead poisoning?
Answer. Sodium calcium edetate and Dimercapol
Question.8. Which therapy is used for lead poisoning?
Answer. Chelation therapy
Question.9. Which antidote is used in the treatment of cyanide poisoning?
Answer. Sodium nitrite, Sodium thiosulphate
Question.10. Differentiate between Physiological antidotes and Mechanical antidotes.
Answer.
- Physiological antidotes- They act by producing the effects opposite to that of poison.
- Mechanical antidotes : They prevent the absorption of poison into body
Antidotes Fill In The Blanks
1. ………….is used to counteract the poison.
Answer: Antidote
2. Cyanide poisoning kit contains . …,………. and ………….
Answer: Amyl nitrite, Sodium nitrite & Sodium thiosulphate
3. Cyanide poisoning has a characteristic odour of ……………….
Answer: Bitter almonds
4………………….therapy is used for lead poisoning.
Answer: Chelation
5. Chemical antidote act by changing the……………..of the poison.
Answer: Chemical nature
6. Activated charcoal is used to…………… the poison.
Answer: Absorb
7…………………is the example of physiological antidote.
Answer: Sodium nitrite
8…………….is the molecular formula of sodium thiosulphate.
Answer: Na2S2O3.5H2O
Antidotes Multiple Choice Questions
1. An important antidote in the treatment of cyanide poison is:
- Ethanol
- Atropine
- Sodium thiosulphate
- Desferrioxamine
Answer: Desferrioxamine
2. In case of poisoning, activated charcoal acts to reduce absorption of the substance by
- Increasing osmotic pressure of the intestinal contents
- Shortening transit time thorugh the gut
- Binding to the poison molecules
- Stimulating the chemoreceptor trigger zone in the medulla
Answer: Binding to the poison molecules
3. When a poison is inhaled what methods you will be thinking of to manage the case?
- Induction of emesis
- Carrying out a gastric lavage
- Thinking of an active elimination technique
- Administration of an antidote
- Both c) and d)
Answer: Both c) and d)
4. An antidote is used
- To counteract the poison
- To enhance the poison
- To cause illness e.g. death
- To prdouce consiousness
Answer: To counteract the poison
5. Which of the following antidotes is not used in cyanide poisoning?
- Hydroxy cobalamine
- Sodium nitrite
- Dimercapol
- Sodium calcium EDTA
Answer: Hydroxycobalamine And Sodium nitrite
6. Which kind of antidote reduces the poison across the intestinal wall?
- Mechanical antidote
- Physiological antidote
- Chemical antidote
- None of the above
Answer: Mechanical antidote
7. Physiological antidotes act by:-
- Absorption of the poison
- Changing the chemical nature of the poison
- Producing the effects opposite to that of poison
- By countering the effects of poison
Answer: Producing the effects opposite to that of poison
8.”Cyanide poisoning kit” contains following:-
- Sodium nitrite
- Amyl nitrite
- Sodium bicarbonate
- Sodium thiosulphate
Answer: Sodium bicarbonate
9. How does mechanical antidote act?
- By producing the effects opposite to that of poison
- By preventing the absorption of poison into the body
- By changing the chemical nature of the poison
- By changing the physical nature of the poison
Answer: By preventing the absorption of poison into the body
10. Which one of the following is an example of Physiological Antidote?
- Sodium nitrite
- Sodium thiocyanate
- Activated charcoal
- Copper sulphate
Answer: Sodium nitrite
11. Cyanide Poisoning has a characteristic odour of
- Bitter almonds
- Wheat
- Lemon
- Caraway
Answer: Bitter almonds
12. Sodium nitrite is given in conjuction with:-
- Sodium bicarbonate
- Sodium thiosulphate
- Sodium calcium edetate
- Dimercaprol
Answer: Sodium thiosulphate
